Question: please answer with correct work, not from simialr problems. will only upvote for correct work and answers! thanks! Problem 2 The reaction of ethyl-tetrabromide with
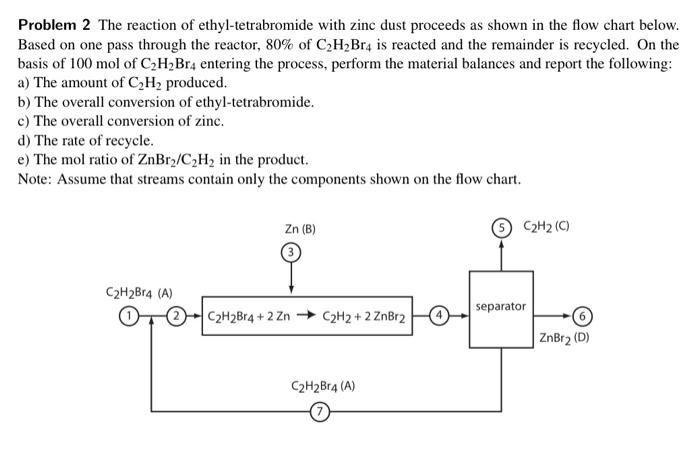
Problem 2 The reaction of ethyl-tetrabromide with zinc dust proceeds as shown in the flow chart below. Based on one pass through the reactor, 80% of C2H2Br4 is reacted and the remainder is recycled. On the basis of 100 mol of C2H_Br4 entering the process, perform the material balances and report the following: a) The amount of C2H2 produced. b) The overall conversion of ethyl-tetrabromide. c) The overall conversion of zinc. d) The rate of recycle. e) The mol ratio of ZnBry/C2H2 in the product. Note: Assume that streams contain only the components shown on the flow chart. Zn (B) C2H2 (0) C2H2B14 (A) separator C2H2Br4+2 Zn C2H2 + 2 ZnBr2 ZnBr2 (D) C2H2Br4 (A)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


