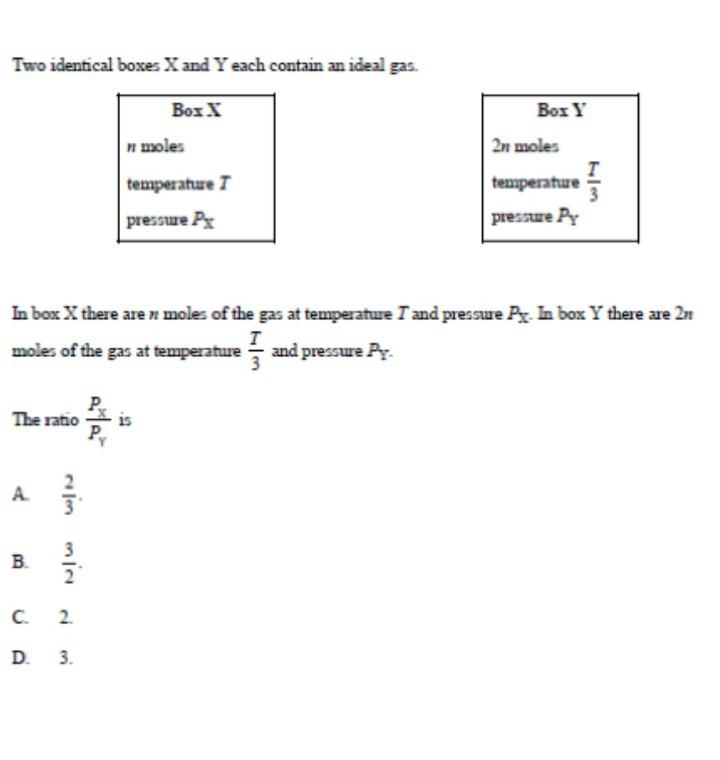
Question: please answer with step clear answer with details Two identical boxes X and Y each contain an ideal gas. begin{tabular}{|l|} hline multicolumn{1}{|c|}{ Box X}
please answer with step clear answer with details
Two identical boxes X and Y each contain an ideal gas. \begin{tabular}{|l|} \hline \multicolumn{1}{|c|}{ Box X} \\ n moles \\ temperature T \\ pressure PX \\ \hline \end{tabular} \begin{tabular}{|l|} \hline \multicolumn{1}{|c|}{ Box Y } \\ 2n moles \\ temperature 3T \\ pressure PY \\ \hline \end{tabular} In bos X there are n moles of the gas at temperature T and pressure P. In box Y there are 2n moles of the gas at temperature 3T and pressure PY - The ratio PYPX is A. 32 B. 23. C. 2 . D. 3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


