Question: please answer with this three questions , thank you so much 1. Which of the following elements has the lowest melting point? A. Sulphur B.
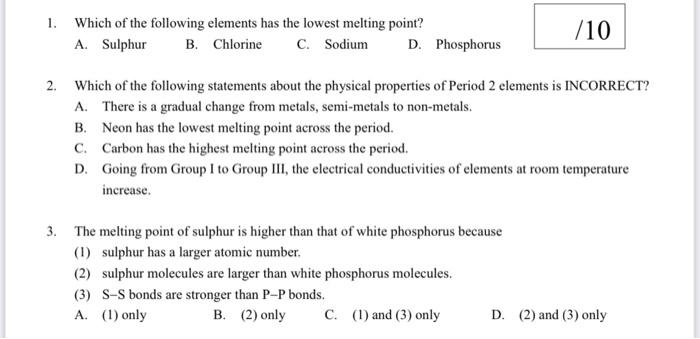
1. Which of the following elements has the lowest melting point? A. Sulphur B. Chlorine C. Sodium D. Phosphorus 2. Which of the following statements about the physical properties of Period 2 elements is INCORRECT? A. There is a gradual change from metals, semi-metals to non-metals. B. Neon has the lowest melting point across the period. C. Carbon has the highest melting point across the period. D. Going from Group I to Group III, the electrical conductivities of elements at room temperature increase. 3. The melting point of sulphur is higher than that of white phosphorus because (1) sulphur has a larger atomic number. (2) sulphur molecules are larger than white phosphorus molecules. (3) SS bonds are stronger than PP bonds. A. (1) only B. (2) only C. (1) and (3) only D. (2) and (3) only
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


