Question: PLEASE ASAP Calculate the pre-exponential term (in s1 ). Answer: The decomposition of formic acid (see below) is measured at several temperatures. HCOOH(g)CO2(g)+H2(g) The temperature
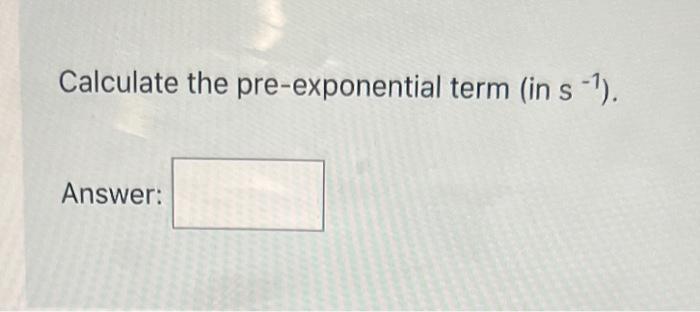

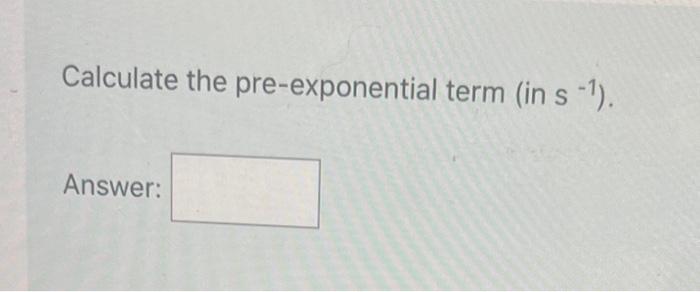
Calculate the pre-exponential term (in s1 ). Answer: The decomposition of formic acid (see below) is measured at several temperatures. HCOOH(g)CO2(g)+H2(g) The temperature dependence of the first-order rate constant is: Calculate the activation elyergy, in kJ/mol. Calculate the pre-exponential term (in s 1 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


