Question: 2) The irreversible liquid phase reaction A B is to be carried out isothermally. The entering flow rate is 100 mol/min of A. The
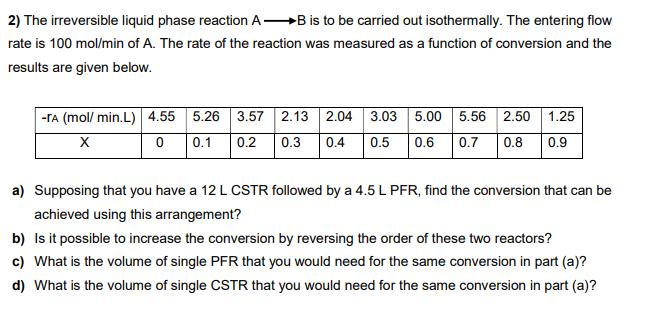
2) The irreversible liquid phase reaction A B is to be carried out isothermally. The entering flow rate is 100 mol/min of A. The rate of the reaction was measured as a function of conversion and the results are given below. -TA (mol/ min.L) 4.55 5.26 3.57 2.13 2.04 3.03 5.00 5.56 2.50 1.25 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 a) Supposing that you have a 12 L CSTR followed by a 4.5 L PFR, find the conversion that can be achieved using this arrangement? b) Is it possible to increase the conversion by reversing the order of these two reactors? c) What is the volume of single PFR that you would need for the same conversion in part (a)? d) What is the volume of single CSTR that you would need for the same conversion in part (a)?
Step by Step Solution
3.29 Rating (152 Votes )
There are 3 Steps involved in it
Answers aThe conversion that can be achieved using this arrangement is ... View full answer

Get step-by-step solutions from verified subject matter experts


