Question: Please complete fully. Thank you! 3.C. Assuming that the CH3 group is sterically larger than the Cl group, rank the rotational states from lowest energy
Please complete fully. Thank you!
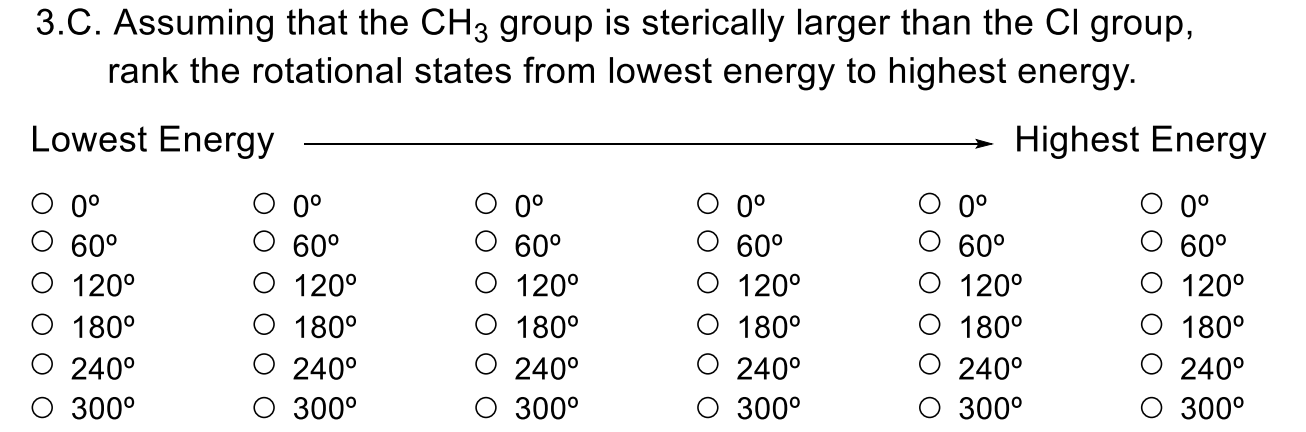
3.C. Assuming that the CH3 group is sterically larger than the Cl group, rank the rotational states from lowest energy to highest energy. Lowest Energy Highest Energy 060120180240300060120180240300060120180240300060120180240300060120180240300060120180240300
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


