Question: Please complete the following: 4. Given that the equilibrium expression may be used to predict the direction of a reaction based on comparisons between Qc
Please complete the following:
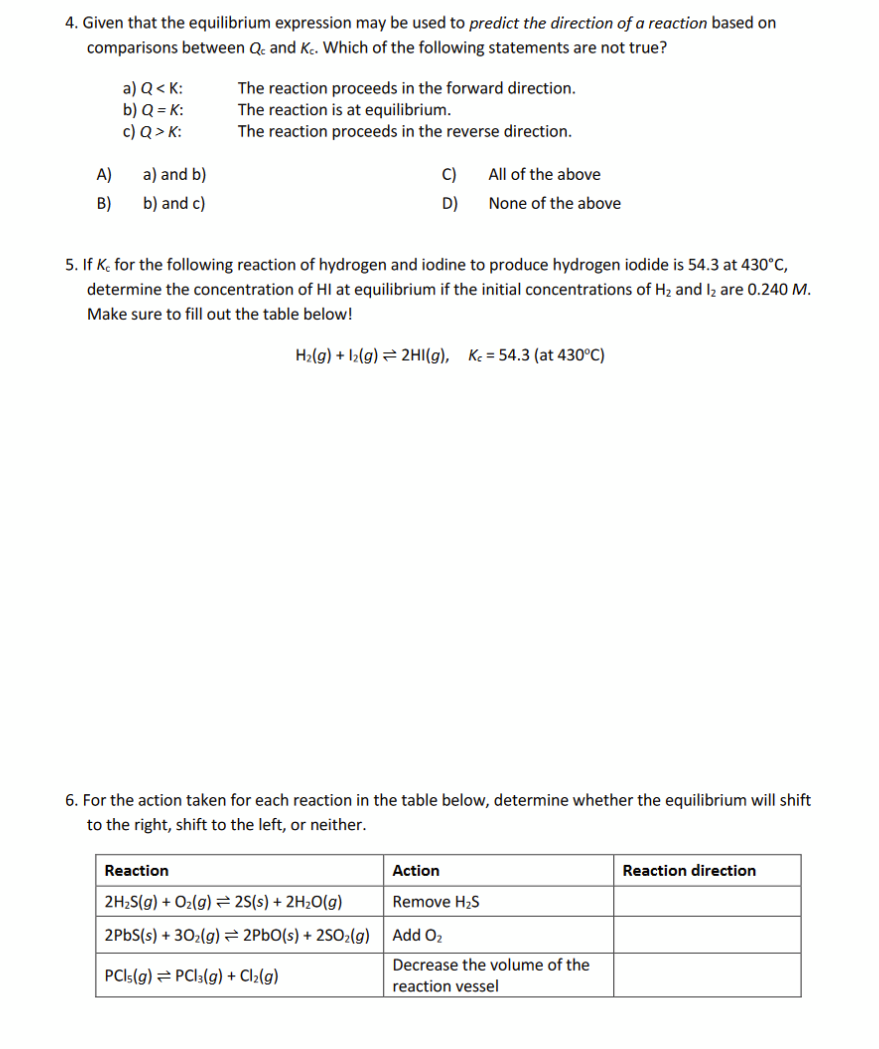
4. Given that the equilibrium expression may be used to predict the direction of a reaction based on comparisons between Qc and Kc. Which of the following statements are not true? a) Q
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


