Question: Please detail the steps behind the solution so I can understand the process. thanks Q # 1. Thermal decomposition of acetone is proposed to follow
Please detail the steps behind the solution so I can understand the process. thanks
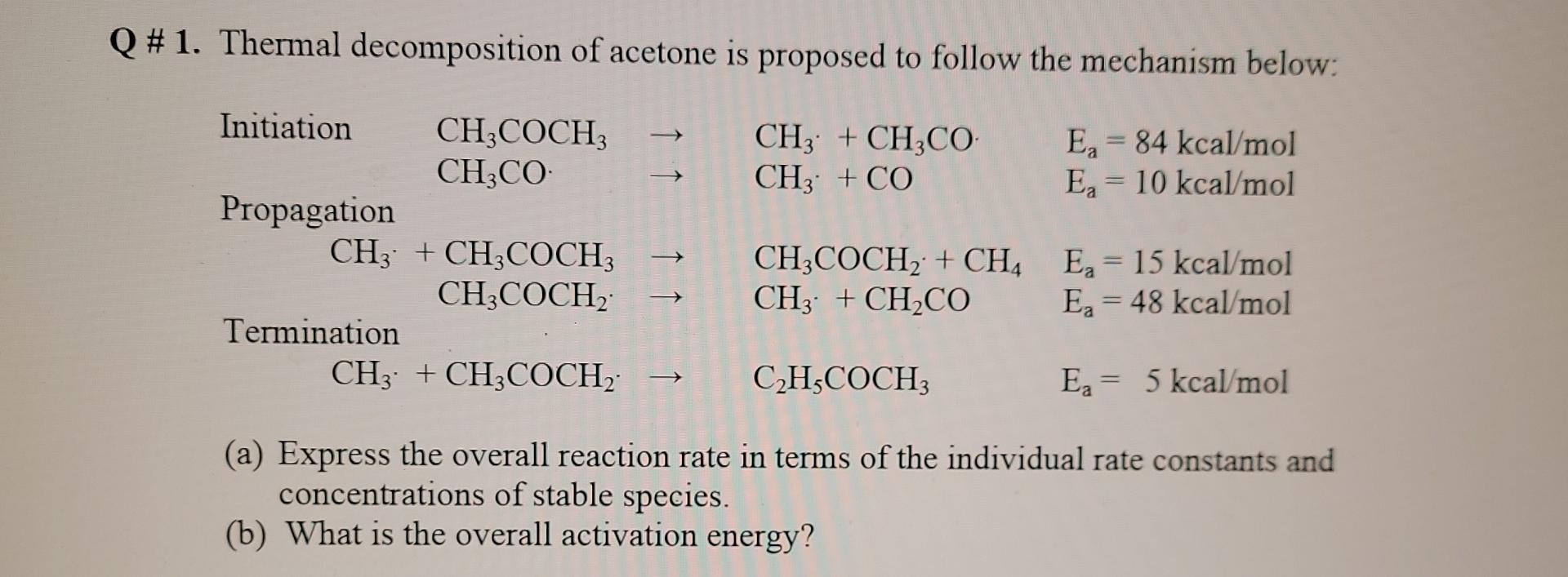
Q # 1. Thermal decomposition of acetone is proposed to follow the mechanism below: CH3 + CH3CO CH3 + CO Ea = 84 kcal/mol Eg = 10 kcal/mol Initiation CH3COCH CH3CO Propagation CH3 + CH3COCH; CH3COCH Termination CH3 + CH3COCH -> CH3COCH2 + CH4 Ex = 15 kcal/mol CH3 + CH CO Ea = 48 kcal/mol - CH3COCH E = 5 kcal/mol (a) Express the overall reaction rate in terms of the individual rate constants and concentrations of stable species. (b) What is the overall activation energy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


