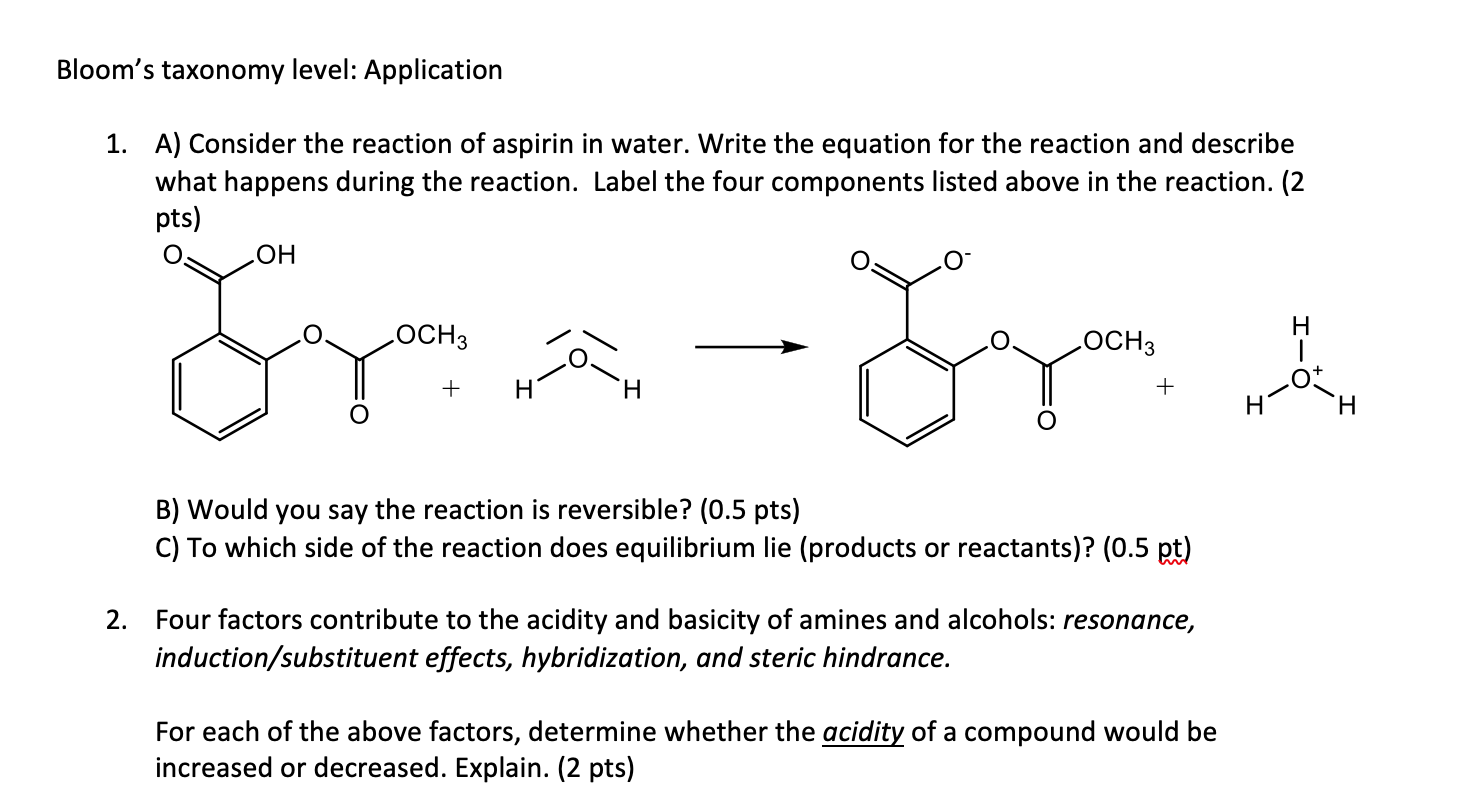
Question: Please do 1 and 2 Bloom's taxonomy level: Application 1. A) Consider the reaction of aspirin in water. Write the equation for the reaction and
 Please do 1 and 2
Please do 1 and 2
Bloom's taxonomy level: Application 1. A) Consider the reaction of aspirin in water. Write the equation for the reaction and describe what happens during the reaction. Label the four components listed above in the reaction. (2 pts) H CYS- OCH3 Gr OCH3 + H H + . B) Would you say the reaction is reversible? (0.5 pts) C) To which side of the reaction does equilibrium lie (products or reactants)? (0.5 pt) 2. Four factors contribute to the acidity and basicity of amines and alcohols: resonance, induction/substituent effects, hybridization, and steric hindrance. For each of the above factors, determine whether the acidity of a compound would be increased or decreased. Explain. (2 pts) Bloom's taxonomy level: Application 1. A) Consider the reaction of aspirin in water. Write the equation for the reaction and describe what happens during the reaction. Label the four components listed above in the reaction. (2 pts) H CYS- OCH3 Gr OCH3 + H H + . B) Would you say the reaction is reversible? (0.5 pts) C) To which side of the reaction does equilibrium lie (products or reactants)? (0.5 pt) 2. Four factors contribute to the acidity and basicity of amines and alcohols: resonance, induction/substituent effects, hybridization, and steric hindrance. For each of the above factors, determine whether the acidity of a compound would be increased or decreased. Explain. (2 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


