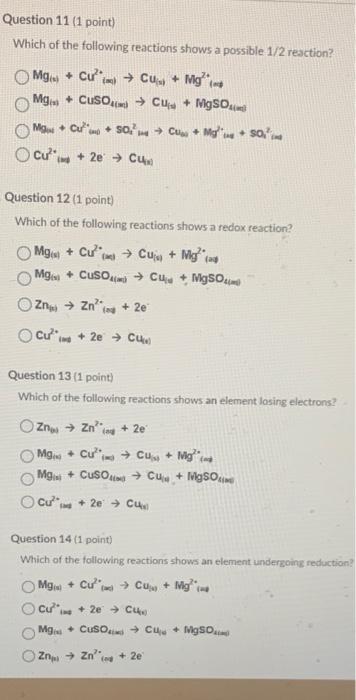
Question: please do all 4 questions, all i need is the answer Which of the following reactions shows a possible 1/2 reaction? Mg(s)+Cu2+(se)Cu(s)+Mg2+(ot)Mg(x)+CuSO4(m)Cu(x)+MgSO4(x)Cu2(se+2eCuw) Question 12 (
 please do all 4 questions, all i need is the answer
please do all 4 questions, all i need is the answerWhich of the following reactions shows a possible 1/2 reaction? Mg(s)+Cu2+(se)Cu(s)+Mg2+(ot)Mg(x)+CuSO4(m)Cu(x)+MgSO4(x)Cu2(se+2eCuw) Question 12 ( 1 point) Which of the following reactions shows a redox reaction? Mg(s)+Cu2(se)Cu(i)+Mg(id)Mg(s)+CuSO4(mij)Cuu+MgSO4maZ2n(t)Zn20(ve+2eCu2+int2+2eCue Question 13 (1 point) Which of the following reactions shows an element losing electrons? Zn6sZ2ias+2ejMg(e)+Cui=j2+Cu(i)+Mgin2Mgisi+CuSO4iaCui+1+MgSOsimeCu21nt+2eCuw Question 14 (1 point) Which of the following reactions shows an element undergoing reduction: Mgia+Cu2(ad)Cu(0)+Mgi=42Cu20in+2eCu10Mgia+CuSOixiCua+FigSOaiajZnpiZn3(nv0+2e
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


