Question: please do all ASAP!! Which would you expect to be larger; H or H? They'd be the same H H 1 point Which would have
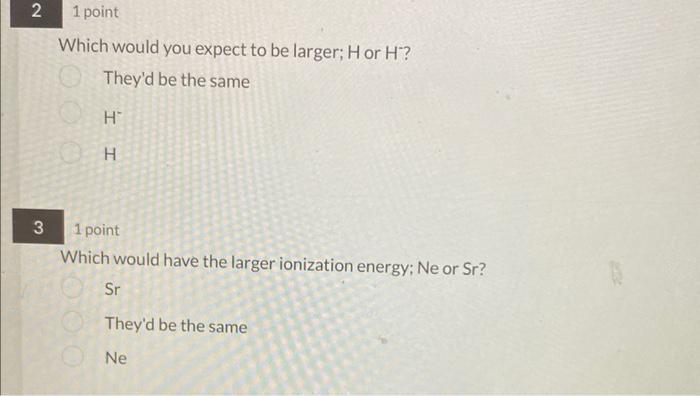

Which would you expect to be larger; H or H? They'd be the same H H 1 point Which would have the larger ionization energy; Ne or Sr? Sr They'd be the same Ne Calculate the net energy change in kJ/mol that takes place on formation of MgF2(s) from the elements: Mg(s)+F2(g)MgF2(s) The following information is needed: Heat of sublimation for Mg(s)=147.7kJ/mol Bond dissociation energy for F2(g)=158kJ/mol Electrostatic interactions in MgF2(s)=2957kJ/mol Ea for F(g)=328kJ/mol E1 for Me(g)=737.7kJ/mol E2 for MB(g)=1450.7kJ/mol 1955 790.9 1119
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


