Question: please do both can you please write out in proportions so that they can be crossed out percent of Ni in the unknown. 4. Using
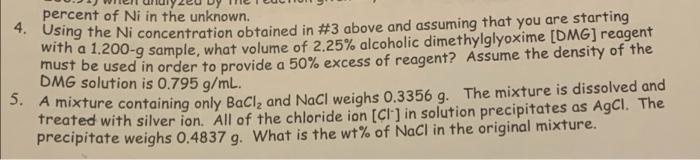

percent of Ni in the unknown. 4. Using the Ni concentration obtained in #3 above and assuming that you are starting with a 1.200-9 sample, what volume of 2.25% alcoholic dimethylglyoxime [DMG] reagent must be used in order to provide a 50% excess of reagent? Assume the density of the DMG solution is 0.795 g/mL. 5. A mixture containing only Ball, and NaCl weighs 0.3356g. The mixture is dissolved and treated with silver ion. All of the chloride ion [Cl] in solution precipitates as AgCl. The precipitate weighs 0.4837 g. What is the wt% of NaCl in the original mixture. 2. A 0,085-9 sunt How many grams of product (MW 206.243) will be forme by the reaction given in your text and in class? 3. A 2.563-9 sample of unknown gave 6.993 g of bis(dimethylglyoximate)nickel(II) (FW 288.91) when analyzed by the reaction given in your text and in class. Find the weight percent of Ni in the unknown. 4. Using the Ni concentration obtained in #3 above and assuming that you are starting with a 1.200-9 sample, what volume of 2.25% alcoholic dimethylglyoxime (DMG) reagent must be used in order to provide a 50% excess of reagent? Assume the density of the ..^705 /ml ne le dicsolved and percent of Ni in the unknown. 4. Using the Ni concentration obtained in #3 above and assuming that you are starting with a 1.200-9 sample, what volume of 2.25% alcoholic dimethylglyoxime [DMG] reagent must be used in order to provide a 50% excess of reagent? Assume the density of the DMG solution is 0.795 g/mL. 5. A mixture containing only Ball, and NaCl weighs 0.3356g. The mixture is dissolved and treated with silver ion. All of the chloride ion [Cl] in solution precipitates as AgCl. The precipitate weighs 0.4837 g. What is the wt% of NaCl in the original mixture. 2. A 0,085-9 sunt How many grams of product (MW 206.243) will be forme by the reaction given in your text and in class? 3. A 2.563-9 sample of unknown gave 6.993 g of bis(dimethylglyoximate)nickel(II) (FW 288.91) when analyzed by the reaction given in your text and in class. Find the weight percent of Ni in the unknown. 4. Using the Ni concentration obtained in #3 above and assuming that you are starting with a 1.200-9 sample, what volume of 2.25% alcoholic dimethylglyoxime (DMG) reagent must be used in order to provide a 50% excess of reagent? Assume the density of the ..^705 /ml ne le dicsolved and
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


