Question: PLEASE DO BOTH ONLY WRITE ANSWER Question 8 (1 point) When a sample of the aqueous layer with a volume of 11.17 mL is collected,
PLEASE DO BOTH ONLY WRITE ANSWER
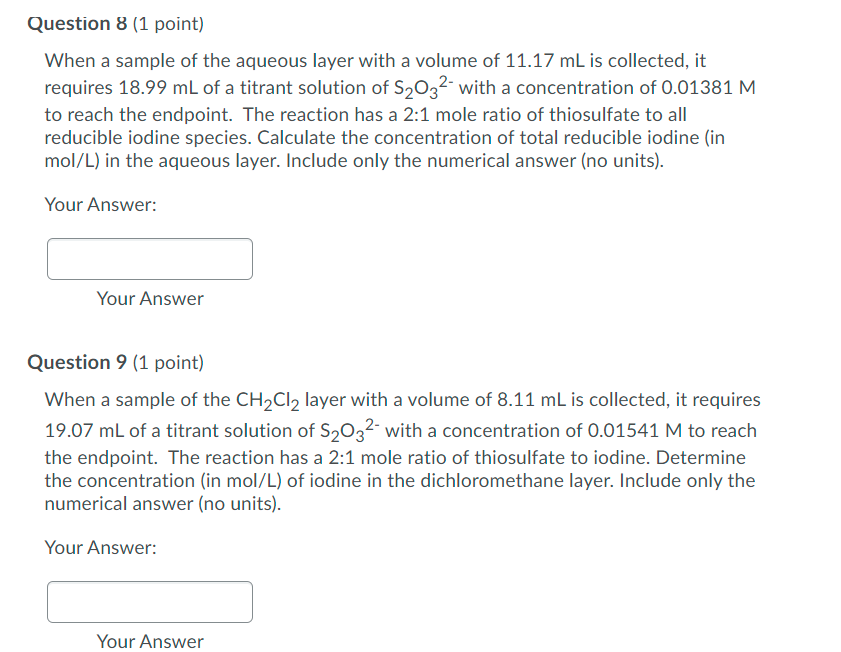
Question 8 (1 point) When a sample of the aqueous layer with a volume of 11.17 mL is collected, it requires 18.99 mL of a titrant solution of S2032- with a concentration of 0.01381 M to reach the endpoint. The reaction has a 2:1 mole ratio of thiosulfate to all reducible iodine species. Calculate the concentration of total reducible iodine (in mol/L) in the aqueous layer. Include only the numerical answer (no units). Your Answer: Your Answer Question 9 (1 point) When a sample of the CH2Cl2 layer with a volume of 8.11 mL is collected, it requires 19.07 mL of a titrant solution of S2032- with a concentration of 0.01541 M to reach the endpoint. The reaction has a 2:1 mole ratio of thiosulfate to iodine. Determine the concentration (in mol/L) of iodine in the dichloromethane layer. Include only the numerical answer (no units). Your Answer: Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


