Question: PLEASE DO EACH PART AND **************DO NOT COPY OTHER ANSWERS FROM CHEGG************** TO RECEIVE A THUMBS UP!!!!!!! 3. Ethyl acetate is an extensively used solvent
PLEASE DO EACH PART AND **************DO NOT COPY OTHER ANSWERS FROM CHEGG************** TO RECEIVE A THUMBS UP!!!!!!!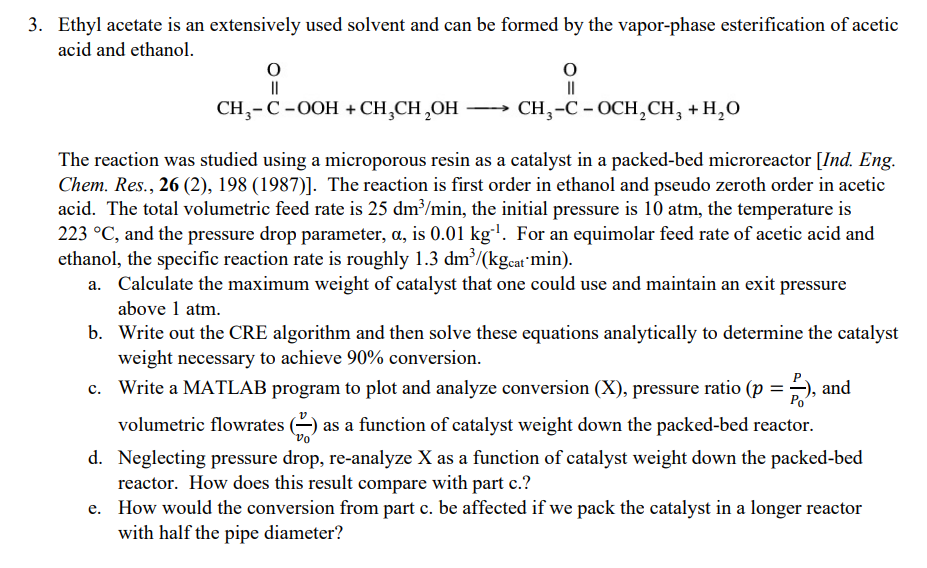
3. Ethyl acetate is an extensively used solvent and can be formed by the vapor-phase esterification of acetic acid and ethanol. 0 0 11 11 CH,-C-OOH + CH,CH,OH - CH, -C -OCH, CH, +H2O The reaction was studied using a microporous resin as a catalyst in a packed-bed microreactor [Ind. Eng. Chem. Res., 26 (2), 198 (1987)]. The reaction is first order in ethanol and pseudo zeroth order in acetic acid. The total volumetric feed rate is 25 dm3/min, the initial pressure is 10 atm, the temperature is 223 C, and the pressure drop parameter, a, is 0.01 kg!. For an equimolar feed rate of acetic acid and ethanol, the specific reaction rate is roughly 1.3 dm/(kgcat'min). a. Calculate the maximum weight of catalyst that one could use and maintain an exit pressure above 1 atm. b. Write out the CRE algorithm and then solve these equations analytically to determine the catalyst weight necessary to achieve 90% conversion. c. Write a MATLAB program to plot and analyze conversion (X), pressure ratio (p = 5), and Po volumetric flowrates as a function of catalyst weight down the packed-bed reactor. d. Neglecting pressure drop, re-analyze X as a function of catalyst weight down the packed-bed reactor. How does this result compare with part c.? e. How would the conversion from part c. be affected if we pack the catalyst in a longer reactor with half the pipe diameter? VO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


