Question: please do explain every step 4. Liquid propane (C3H8) enters inside of a combustion chamber at 25C at a rate of 0.05kg/min where it mixed
please do explain every step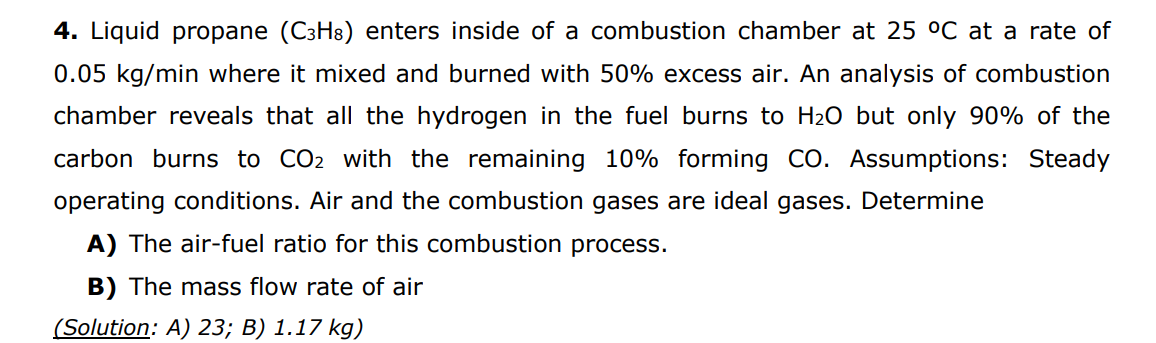
4. Liquid propane (C3H8) enters inside of a combustion chamber at 25C at a rate of 0.05kg/min where it mixed and burned with 50% excess air. An analysis of combustion chamber reveals that all the hydrogen in the fuel burns to H2O but only 90% of the carbon burns to CO2 with the remaining 10% forming CO. Assumptions: Steady operating conditions. Air and the combustion gases are ideal gases. Determine A) The air-fuel ratio for this combustion process. B) The mass flow rate of air (Solution: A) 23;B)1.17kg )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


