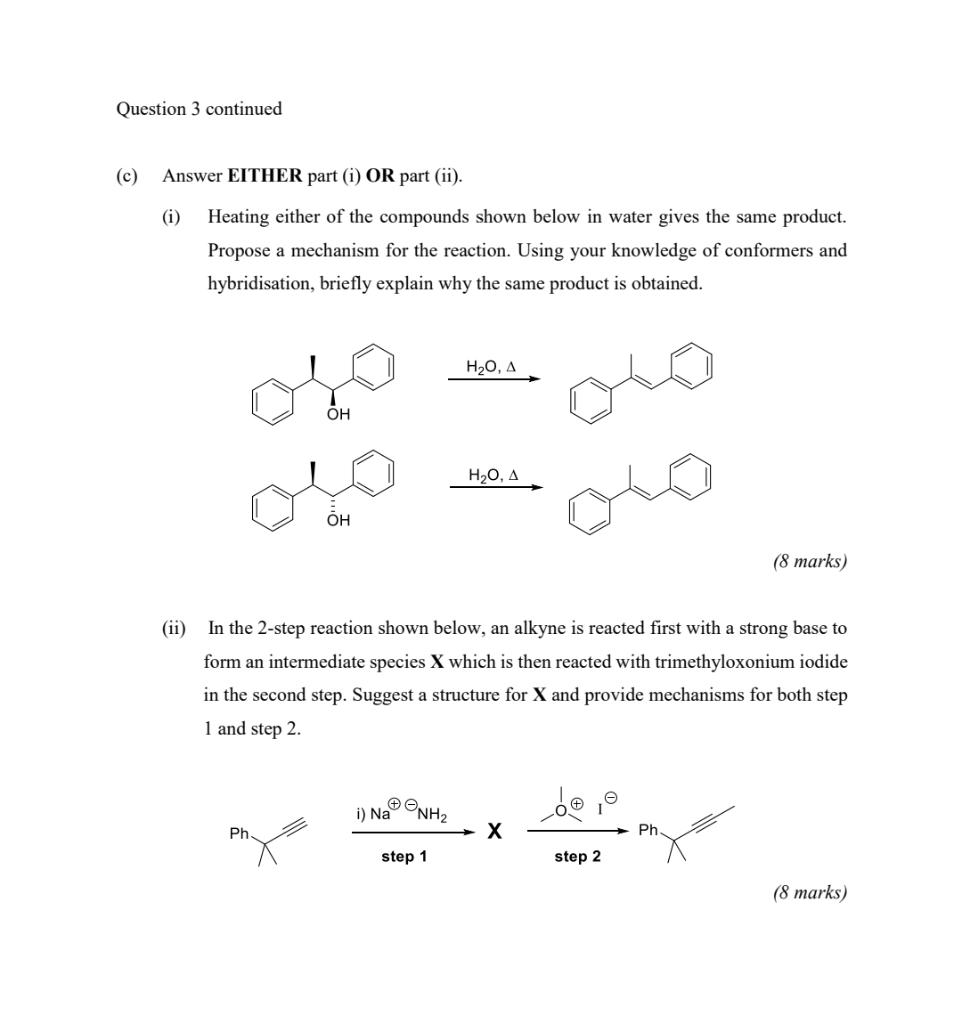
Question: please do fast Question 3 continued Answer EITHER part (i) OR part (ii). (i) Heating either of the compounds shown below in water gives the
 please do fast
please do fast
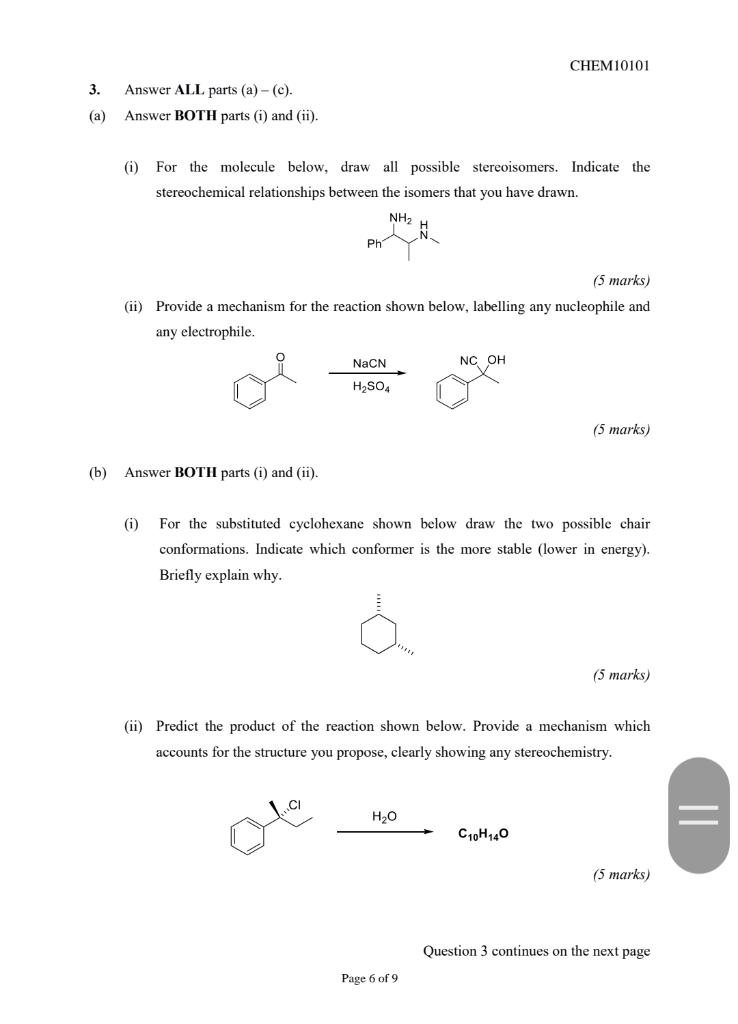
Question 3 continued Answer EITHER part (i) OR part (ii). (i) Heating either of the compounds shown below in water gives the same product. Propose a mechanism for the reaction. Using your knowledge of conformers and hybridisation, briefly explain why the same product is obtained. H20, A OH H20, 4 OH (8 marks) (ii) In the 2-step reaction shown below, an alkyne is reacted first with a strong base to form an intermediate species X which is then reacted with trimethyloxonium iodide in the second step. Suggest a structure for X and provide mechanisms for both step 1 and step 2 ONH2 i) Na Ph X Ph step 1 step 2 (8 marks) CHEM10101 3. Answer ALL parts (a)-(c). Answer BOTH parts (i) and (ii). (a) (i) For the molecule below, draw all possible stereoisomers. Indicate the stereochemical relationships between the isomers that you have drawn. NH2 Ph (5 marks) (ii) Provide a mechanism for the reaction shown below, labelling any nucleophile and any electrophile. NaCN NC OH H2SO4 (5 marks) (b) Answer BOTH parts (i) and (ii). (0) For the substituted cyclohexane shown below draw the two possible chair conformations. Indicate which conformer is the more stable (lower in energy). Briefly explain why. (5 marks) (ii) Predict the product of the reaction shown below. Provide a mechanism which accounts for the structure you propose, clearly showing any stereochemistry. H2O = C10H140 (5 marks Question 3 continues on the next page Page 6 of 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


