Question: please do not copy from previous chegg problems and this are new values. thank you i will upvote The specific internal energy of formaldehyde (HCHO)
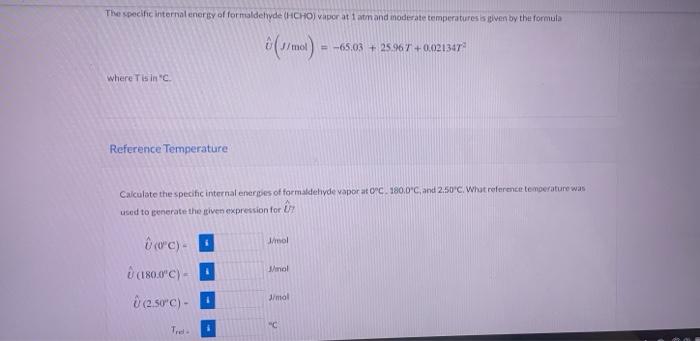
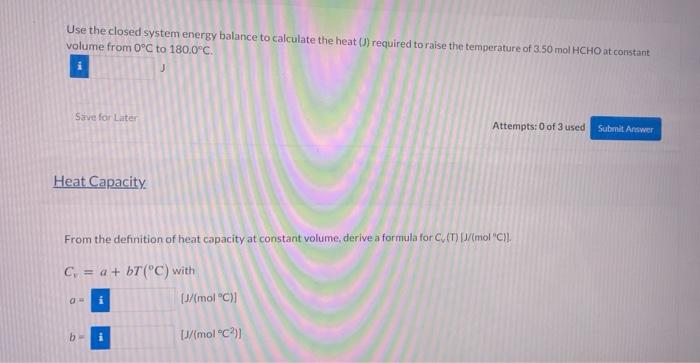
The specific internal energy of formaldehyde (HCHO) vapor ati atm and inoderate temperatures is oven by the formula (1) mot) = -65,03 + 25.967 +0.021347 where Tisin Reference Temperature Calculate the specific internal energies of formaldehyde vapor ato C. 180.0C, and 2.50'C. What reference temperature was used to generate the given expression for Nool Whol 0 (0C). (180.00) esc) - J/mol "C Tre Use the closed system energy balance to calculate the heat )) required to raise the temperature of 3.50 mol HCHO at constant volume from 0C to 180.0C. Save for Later Attempts: 0 of 3 used Submit Answer Heat Capacity From the definition of heat capacity at constant volume derive a formula for CD/mol "C). C = a + bT(C) with [J/mol C)] b DJ/mol C1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


