Question: PLEASE DO NOT COPY PREVIOUS ANSWERS, I NEED A DIFFERENT EXPLANATION One has a non-ideal liquid mixture of propane and trichloromethane. At 35.0 C the
PLEASE DO NOT COPY PREVIOUS ANSWERS, I NEED A DIFFERENT EXPLANATION
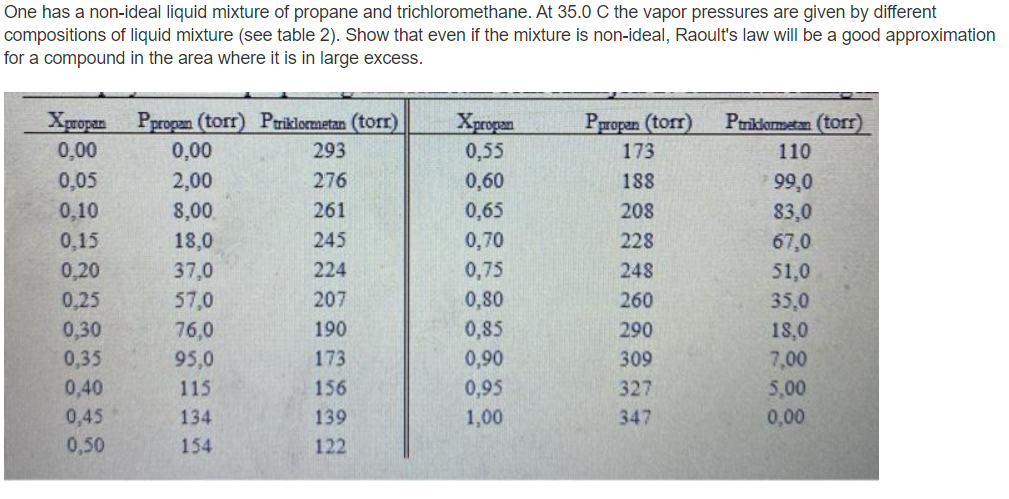
One has a non-ideal liquid mixture of propane and trichloromethane. At 35.0 C the vapor pressures are given by different compositions of liquid mixture (see table 2). Show that even if the mixture is non-ideal, Raoult's law will be a good approximation for a compound in the area where it is in large excess. Xpropian Xpropan 0,00 0,05 0,10 0.15 0,20 0.25 0.30 0,35 0,40 0,45 0,50 Ppropan (torr) Priklormetan (tor) 0,00 293 2,00 276 8,00 261 18,0 245 37,0 224 57,0 207 76,0 190 95,0 173 115 156 134 139 154 122 0,55 0,60 0,65 0,70 0,75 0,80 0,85 0,90 0,95 1,00 Ppropan (tor) 173 188 208 228 248 260 290 309 327 347 Ptriklormatan (torr) 110 99,0 83,0 67,0 51,0 35.0 18,0 7,00 5,00 0,00
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


