Question: (PLEASE DO NOT COPY YOUR ANSWER FROM A DIFFERENT QUESTION AND POST IT HERE. PLEASE READ THE QUESTION CAREFULLY. THIS QUESTION IS DIFFERENT FROM OTHER
(PLEASE DO NOT COPY YOUR ANSWER FROM A DIFFERENT QUESTION AND POST IT HERE. PLEASE READ THE QUESTION CAREFULLY. THIS QUESTION IS DIFFERENT FROM OTHER QUESTIONS ON CHEGG. IF THE SAME ANSWER AS OTHER QUESTIONS IS POSTED THERE WILL BE REPORTS AND DISLIKES. PLEASE AVOID IT.)
t-Butyl alcohol (TBA) is an important octane enhancer that is used to replace lead additives in gasoline. TBA was produced by the liquid-phase hydration (W - water) of isobutene (I) over an Amberlyst-15 catalyst.
Assume the reaction proceeds as follows
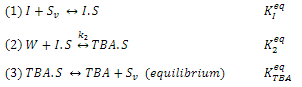
Where Sv represents vacant sites on the surface of the catalyst. The surface reaction (step 2) is rate- limiting. The adsorption of isobutene and TBA comply with the 2 component Langmuir equation. Adsorption equilibrium is assumed.
Derive a rate law for TBA production in the absence of diffusion limitations as a function of , and in solution.
(1)1+S, 1.5 K,99 ka KP (2) W +1.5 TBAS (3) TBA.S TBA + 5, (equilibrium) 499 TBA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


