Question: Please do not skip the explanation step by step. Thank you:) The reaction A+BC+Drate=k[A][B]2 has an initial rate of 0.0340M/s. What will the initial rate
Please do not skip the explanation step by step. Thank you:)
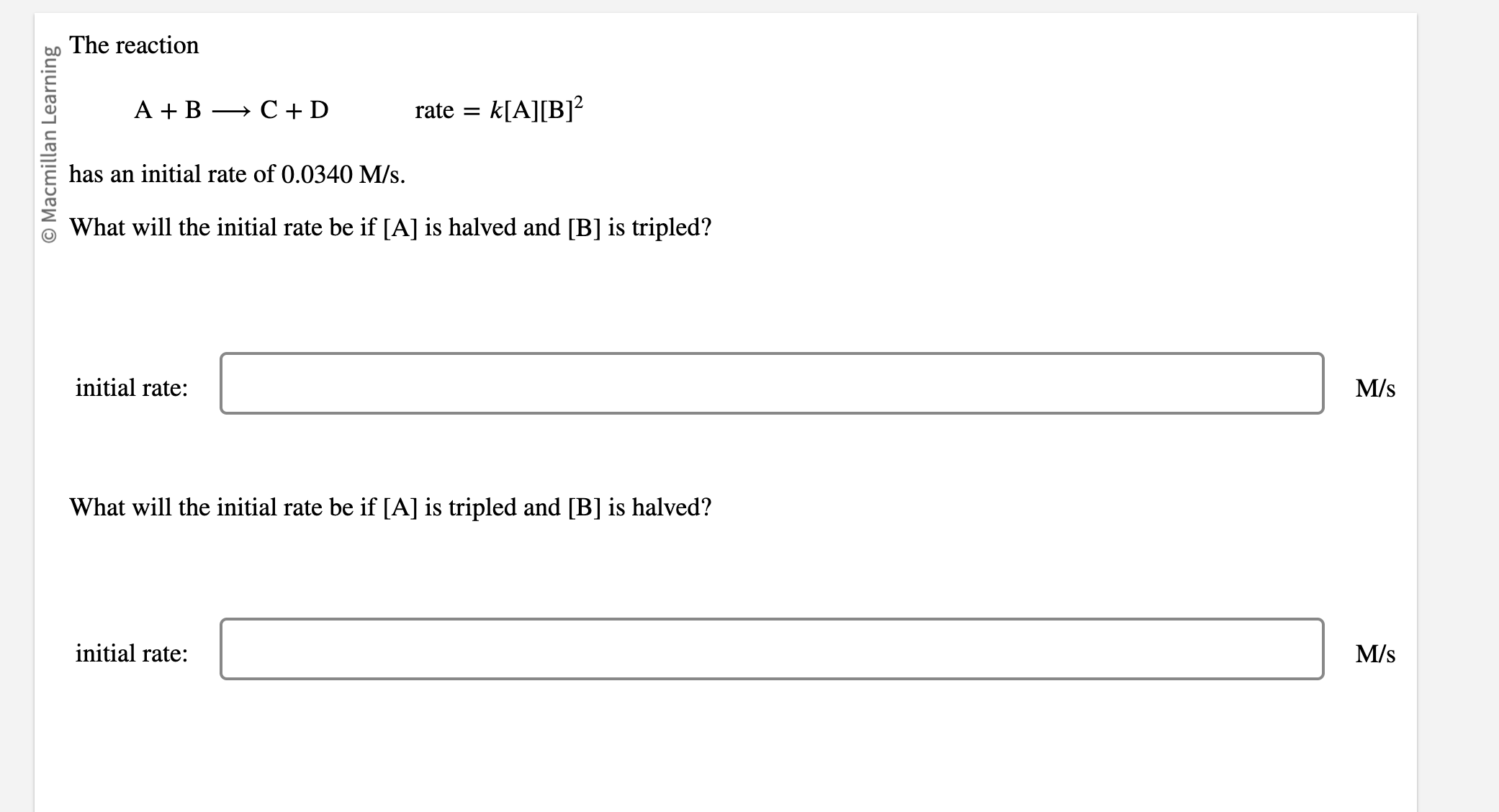
The reaction A+BC+Drate=k[A][B]2 has an initial rate of 0.0340M/s. What will the initial rate be if [A] is halved and [B] is tripled? initial rate: What will the initial rate be if [A] is tripled and [B] is halved? initial rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


