Question: PLEASE DO NOT solve using fuller etal method. EXAMPLE 2.10 (Diffusion of only one component in a three-component mixture) A mixture of 60% hydrogen and
PLEASE DO NOT solve using fuller etal method.
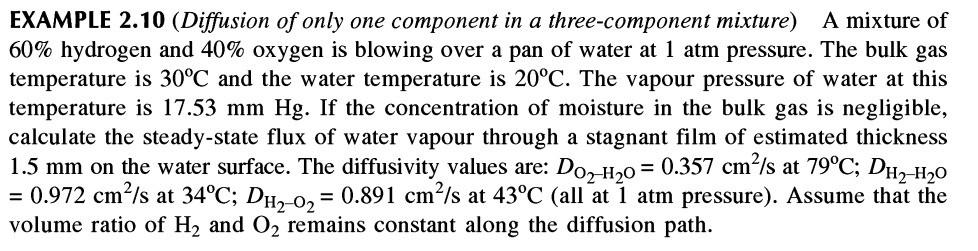
EXAMPLE 2.10 (Diffusion of only one component in a three-component mixture) A mixture of 60% hydrogen and 40% oxygen is blowing over a pan of water at 1 atm pressure. The bulk gas temperature is 30C and the water temperature is 20C. The vapour pressure of water at this temperature is 17.53 mm Hg. If the concentration of moisture in the bulk gas is negligible, calculate the steady-state flux of water vapour through a stagnant film of estimated thickness 1.5 mm on the water surface. The diffusivity values are: Do2-H20 = 0.357 cm/s at 79C; DH2-H20 = 0.972 cmls at 34C; Dh2-02 = 0.891 cm/s at 43C (all at atm pressure). Assume that the volume ratio of H2 and O2 remains constant along the diffusion path. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


