Question: please do the question on the last page including the percent error. thanks Minimize 2 Unit Part A Mass of acetylsalicylic acid data and molar
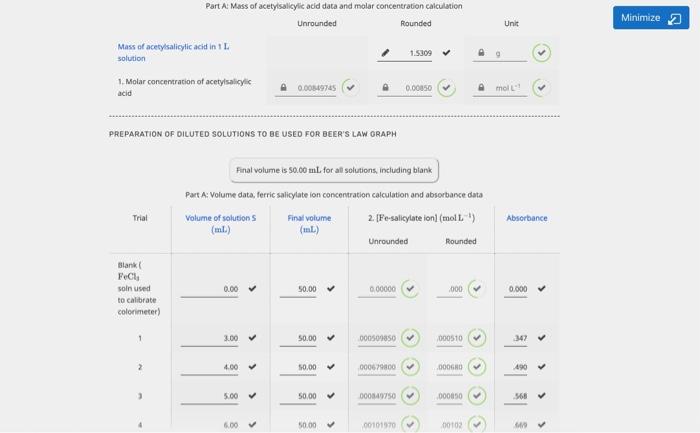
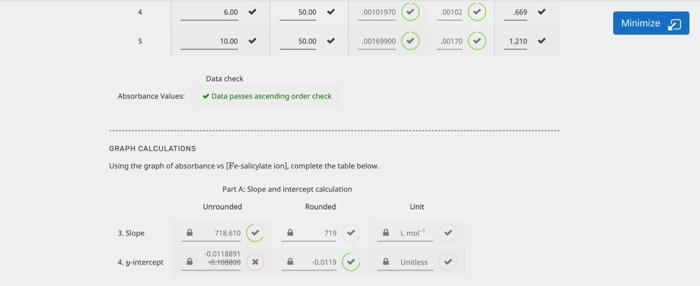
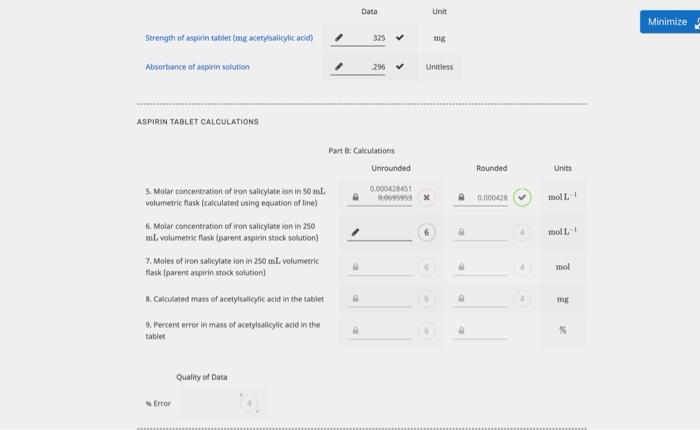
Minimize 2 Unit Part A Mass of acetylsalicylic acid data and molar concentration calculation Unrounded Rounded Mass of acetylsalicylic acid in 1 solution 1.5309 1. Molar concentration of acetylsalicylic acid 0.00849745 0.00350 mol PREPARATION OF DILUTED SOLUTIONS TO BE USED FOR BEER'S LAW GRAPH Final volume is 50.00 mL for all solutions, including blank Part A: Volume data, ferric salicylate on concentration calculation and absorbance data Volume of solutions Final volume 2. [Fe-salicylate ion) (mol L.) (ml) (L.) Unrounded Rounded Trial Absorbance Blank Fel soln used to calibrate colorimeter) 0.00 50.00 0.00000 000 0.000 > 3.00 50.00 > 000509850 000510 > 347 > 2 4.00 > 50.00 000679800 00060 > 490 > 5.00 > 50.00 000140750 000050 > 5468 > 6.00 50.00 > 00101970 00102 6.00 50.00 > 00101970 00102 > 5669 > Minimize 2 10.00 50.00 0016900 00170 1.210 Data check Absorbance Values Data passes ascending order check GRAPH CALCULATIONS Using the graph of absorbance vs [Fe-salicylate ion complete the table below. Part A Slope and intercept calculation Unrounded Rounded Unit 3. Slope 718.610 719 Limon 4. y-intercept -0.0118891 --060 -0.0119 Untless Data Unit Minimize Strength of aspirin tablet (mg acetylsalicylic acid) 325 mg Absorbance of aspirin solution 296 Unitless ASPIRIN TABLET CALCULATIONS Rounded Units 0.000420 mol-1 moll Part : Calculations Unrounded 5. Molar concentration of iron salicylate lon in 50 ml 0.000428451 0.009595 x volumetric flask (calculated using equation of line) 6. Molar concentration of iron salicylate ion in 250 mL volumetric Rask (parent aspirin stock solution) 7. Moles of iron salicylate son in 250 ml. volumetric flask (parent aspirin stock solution) Calculated mass of acetylsalicylic acid in the tablet Percent error in mass of acetylsalicylic acid in the tablet mol mg x Quality of Data Error
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


