Question: 1. Using the VSEPR approach to molecular geometry, predict the shape of each of the species. (5 pt) (Considering only atoms, not electrons) (a) PH3


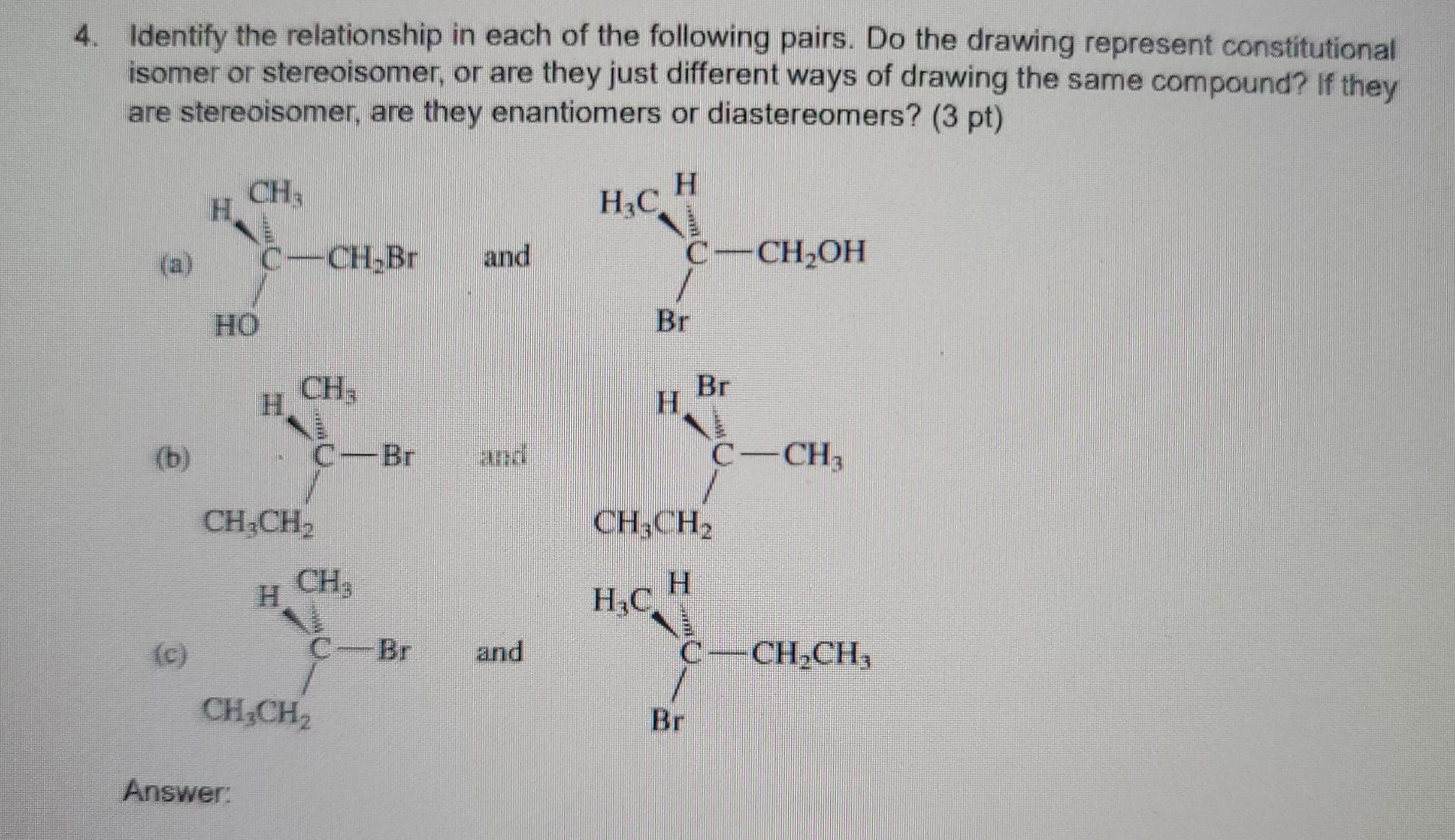




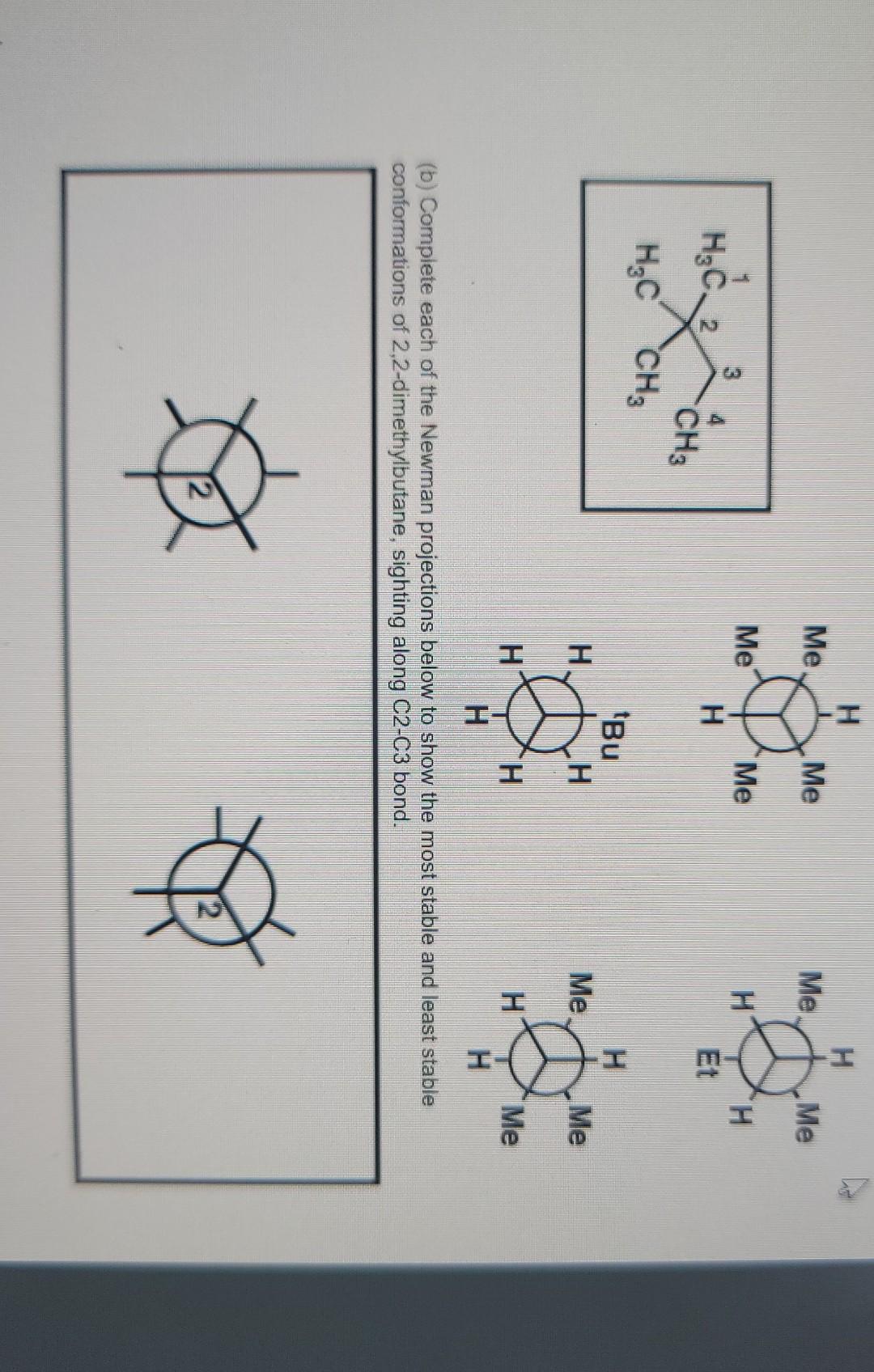
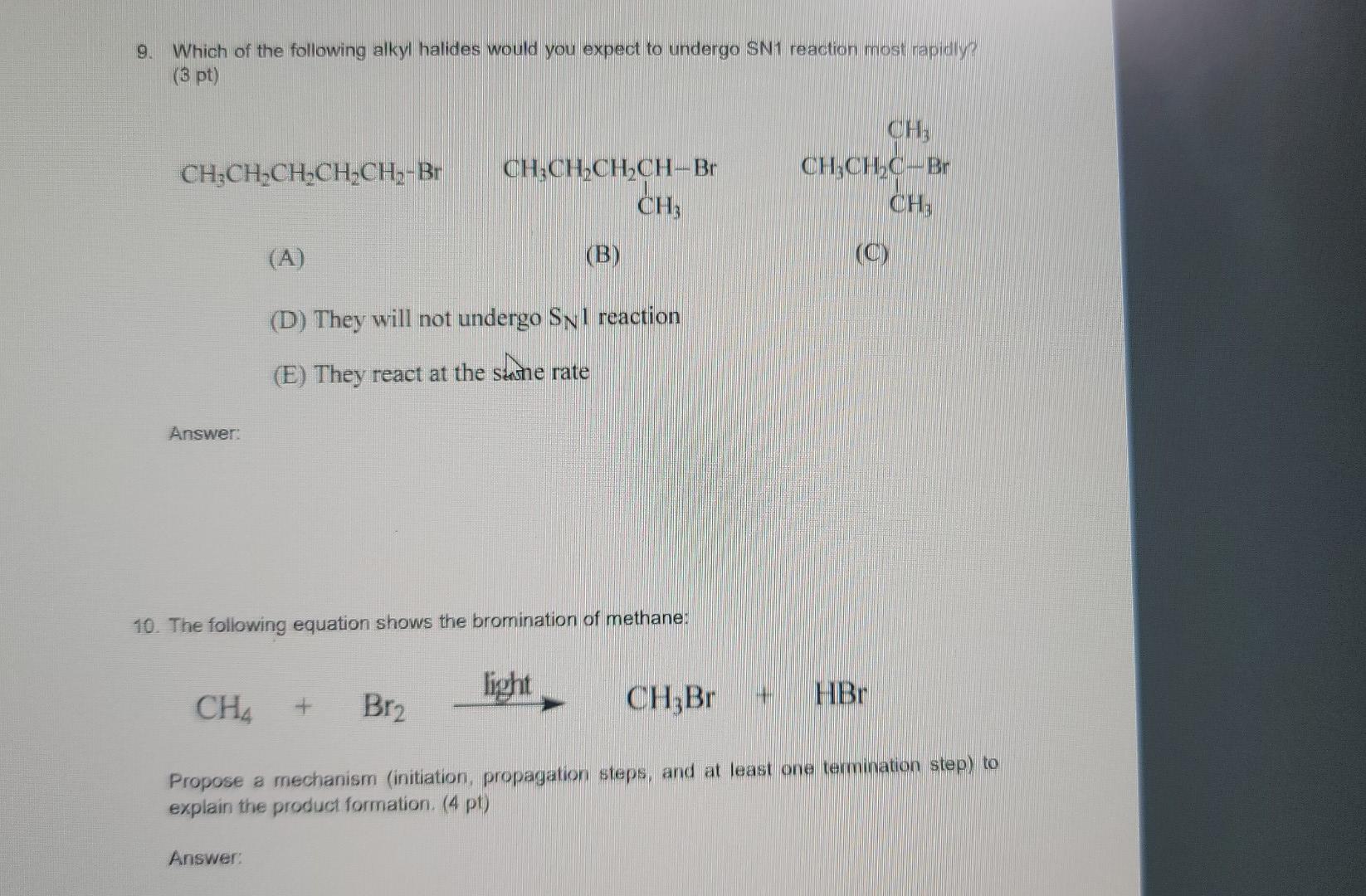

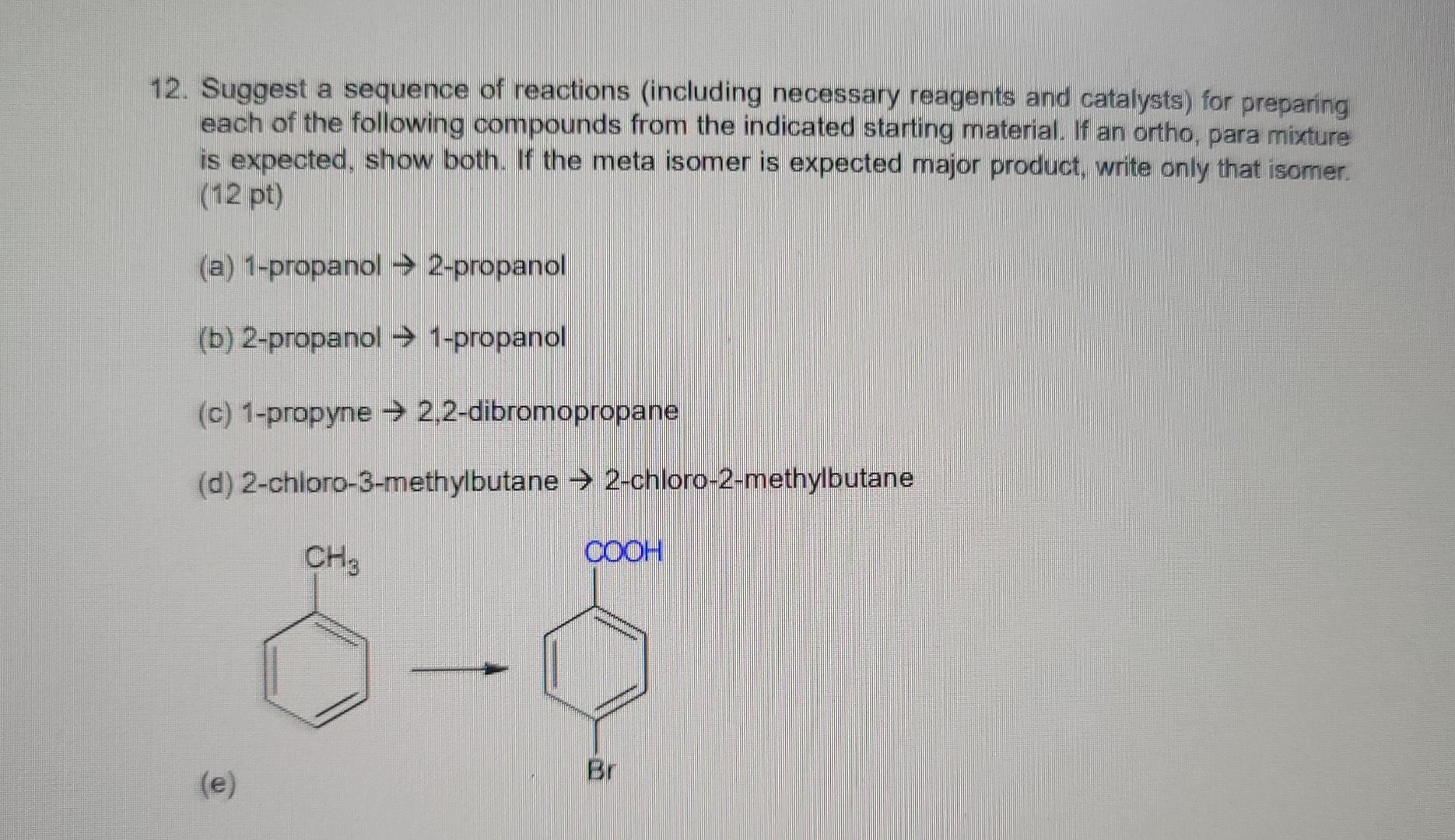
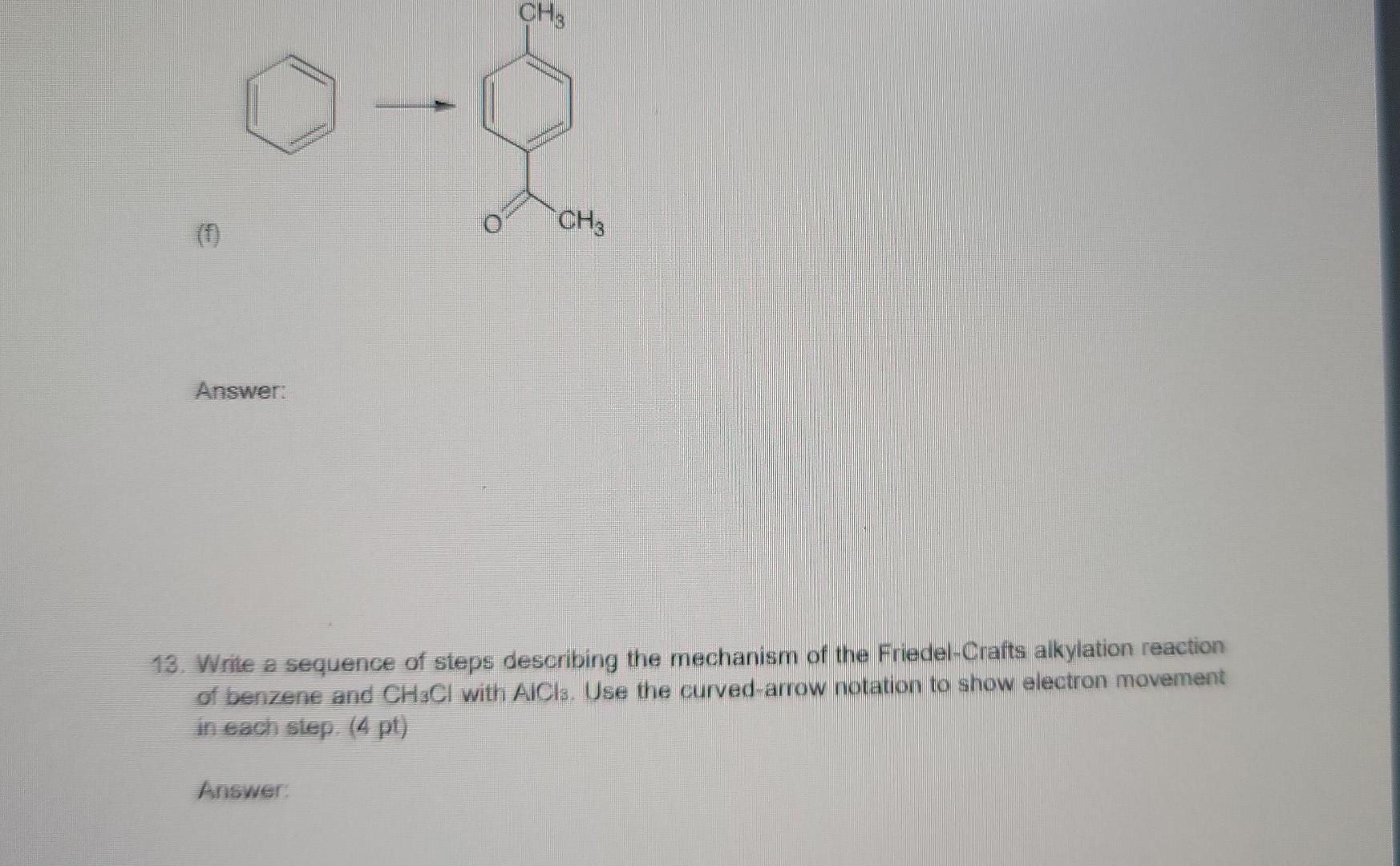
1. Using the VSEPR approach to molecular geometry, predict the shape of each of the species. (5 pt) (Considering only atoms, not electrons) (a) PH3 (b) ATH (c) CoCl2 (all atoms bonded to carbon) (d) HCO3- (e) NO2+ (order of atoms is ONO) Answer: 2. If a chiral molecule has an absolute configuration of R, which direction does it rotate the of polarized light? (2 pt) (a) clockwise (b) counter-clockwise (c) it does not rotate the plane of polarized light Answer: 3. For each of the following pairs of ions, identify the stronger base: (3 pt) (a) OH or NHz (b) For CH (c) CHOCHZO or CH3C00- Answer 4. Identify the relationship in each of the following pairs. Do the drawing represent constitutional isomer or stereoisomer, or are they just different ways of drawing the same compound? If they are stereoisomer, are they enantiomers or diastereomers? (3 pt) CH H H.C. C-CHOH (a) _CH.Br and Br CH: (b) Br CCH, CH,CH, CH3CH2 CH, HC H . (c) C-Br and C-CH.CH CH2CH2 Br Answer: 5. For each molecule, draw the two possible chair conformers and choose the preferred (more stable) conformer. (4 pt) a) cis-1,3-dimethylcyclohexane b) trans-1,4-dimethylcyclohexane between the two chair conformers is ) c The energy difference part a than in part b. Why? greater in 6. Write a sequence of steps describing the mechanism of the acid-catalyzed dehydration of 3- methyl-2-butanol. Use the curved-arrow notation to show electron movement in each step. (4 pt) Answer: 7. Write the structure of the major organic product or products formed on treatment of 1- methylcyclohexene with each of the following: (6 pt) (a) hydrogen bromide (b) bromine (c) bromine in water Answer 8. (6 pt) (a) Circle the structure that represents a conformation of 2,2-dimethylbutane sighting along any C-C bond. (tBu is tertiary butyl) H H Me Me Me Me Me Me 3 H H H HC. 2 H CH3 . H tBu . Me Me H H H Me H H (b) Complete each of the Newman projections below to show the most stable and least stable conformations of 2,2-dimethylbutane, sighting along C2-C3 bond. 9. Which of the following alkyl halides would you expect to undergo SN1 reaction most rapidly? (3 pt) CH3CH2CH2CH_CH2-Br CH3CH2CH2CH-Br CH, CH CH2CH.C-Br CH; (A) (B) (D) They will not undergo Snl reaction (E) They react at the same rate shne Answer: 10. The following equation shows the bromination of methane: light H + CHBr Br2 HBr Propose a mechanism (initiation, propagation steps, and at least one termination step) to explain the product formation. (4 pt) Answer: 11. Choose the molecule containing more acidic hydrogen (4 pt) (a) H2O vs NH3 (b) HCI vs HI (c) NH3 VS NH" (d) C2H2 vs CHA Answer: 12. Suggest a sequence of reactions (including necessary reagents and catalysts) for preparing each of the following compounds from the indicated starting material. If an ortho, para mixture is expected, show both. If the meta isomer is expected major product, write only that isomer. (12 pt) (a) 1-propanol 2-propanol (b) 2-propanol 1-propanol (c) 1-propyne 2,2-dibromopropane (d) 2-chloro-3-methylbutane 2-chloro-2-methylbutane CH3 COOH Br (e) e CH3 CH3 (0 Answer: 13. Write a sequence of steps describing the mechanism of the Friedel-Crafts alkylation reaction of benzene and CH3CI with AlCl3. Use the curved-arrow notation to show electron movement in each step. (4 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


