Question: please do them correctly According to the following reaction, how many grams of carbon dioxide will be formed upon the complete reaction of 22.6 grams
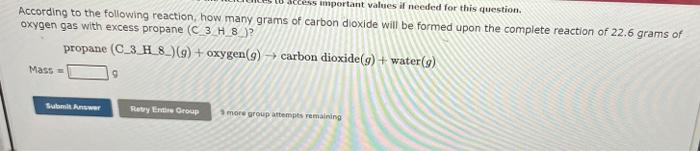



According to the following reaction, how many grams of carbon dioxide will be formed upon the complete reaction of 22.6 grams of oxygen gas with excess propane (C3H/8)2 ? \[ \begin{array}{l} \text { propane }(\text { C_3_H_8_ }(g)+\operatorname{oxygen}(g) ightarrow \operatorname{carbon} \operatorname{dioxide}(g)+\operatorname{water}(g) \\ \text { Mass = } \end{array} \] S mere group attempts remaining Use the Rielerences to access impartant values if needed for this question. According to the foliowing reaction, how many grams of carbon tetrachloride are required for the complete reaction of 24.8 grams of methene (CH4) ? methase (CH4)(g)+ carbon tetrachloride (g) dichlorotuethuse (CH4Cl2)(g) Mins = grams carben tetrachionde For the following reaction, 3.53 grams of propane (C3HB) are mixed with excess oxygen gas. The reactiont yields: 7.07 grams of carbon dioxide. propane(C3Hs)()+oxygen()-carbondioxide()+water() What is the thenretical yieid of carbon aloxide ? What is the percent yiela of carbon dioxide? For the foliowing reaction, 4.30 grams of water are mixed with excess carbon monoxide. The reaction yields 7.93 grams of carbon dioxide. carbon monoxide (g)+ water (1) carbon dioxide (g) + hydrogen (g) What is the theoretical vield of carbon dioxide? What is the percent veld of carbon dioxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


