Question: please answer 4 pages Use the References to access important values if needed for this question. According to the following reaction, how many grams of
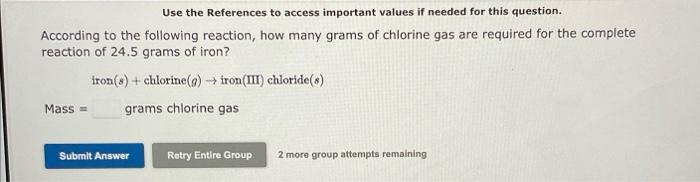



Use the References to access important values if needed for this question. According to the following reaction, how many grams of chlorine gas are required for the complete reaction of 24.5 grams of iron? iron(s)+chlorine(g)iron(III)chloride(s)Mass=gramschlorinegas Use the References to access important values if needed for this question. According to the following reaction, how many grams of carbon dioxide will be formed upon the complete reaction of 27.2 grams of oxygen gas with excess carbon (graphite)? Mass=carbon(graphite)(g)+axygen(g)carbondioxide(g)gramscarbondioxide Use the References to access important values if needed for this question. According to the following reaction, how many grams of water will be formed upon the complete reaction of 31.9 grams of hydrogen peroxide (H2O2) ? hydrogen peroxide (H2O2)(aq) water (I)+oxygen(g) grams water Use the References to access important values if needed for this question. When carbon disulfide reacts with chlorine, carbon tetrachloride and sulfur dichloride are produced. The balanced equation for this reaction is: CS2(s)+4Cl2(a)CCl4(l)+2SCl2(s) If 3 moles of carbon disulfide react, The reaction consumes moles of chlorine. The reaction produces moles of carbon tetrachloride and moles of sulfur dichloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


