Question: Please do this in MATLAB, thank you. 2. N204 is decomposed into 2NO2 in gas phase. The equilibrium extent of decomposition X is given by
 Please do this in MATLAB, thank you.
Please do this in MATLAB, thank you.
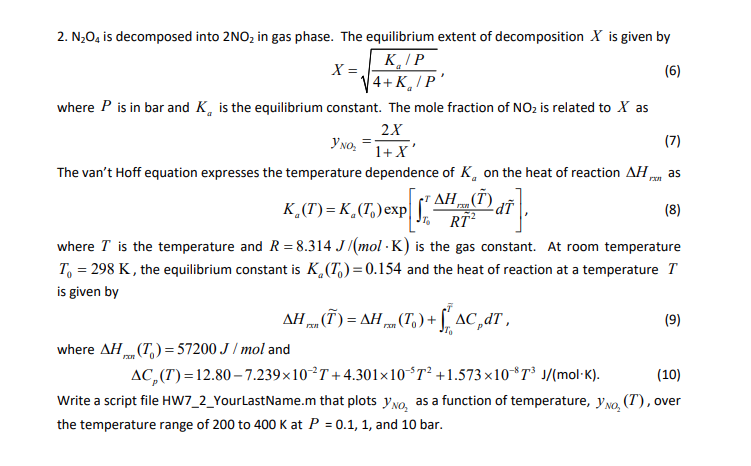
2. N204 is decomposed into 2NO2 in gas phase. The equilibrium extent of decomposition X is given by KIP X= (6) 14+K/P' where P is in bar and K, is the equilibrium constant. The mole fraction of NO2 is related to X as 2x , (7) 1+X' The van't Hoff equation expresses the temperature dependence of K, on the heat of reaction AH, as PAH_(I) d K_(7)=K_(7.)exp , ( Dail . 8 7 (8) RT- where T is the temperature and R = 8.314 J/mol K) is the gas constant. At room temperature To = 298 K, the equilibrium constant is K.(T.)=0.154 and the heat of reaction at a temperature T is given by AH _o()= a . (T.) +% 40,dr, (9) where AH.(T) = 57200 J/mol and AC,(T)=12.80 7.239x10-T +4.301x10-$T? +1.573x108T J/mol-K). Write a script file HW7_2_YourLastName.m that plots yno, as a function of temperature, yno, (T), over the temperature range of 200 to 400 K at P = 0.1, 1, and 10 bar. ) (10) 2. N204 is decomposed into 2NO2 in gas phase. The equilibrium extent of decomposition X is given by KIP X= (6) 14+K/P' where P is in bar and K, is the equilibrium constant. The mole fraction of NO2 is related to X as 2x , (7) 1+X' The van't Hoff equation expresses the temperature dependence of K, on the heat of reaction AH, as PAH_(I) d K_(7)=K_(7.)exp , ( Dail . 8 7 (8) RT- where T is the temperature and R = 8.314 J/mol K) is the gas constant. At room temperature To = 298 K, the equilibrium constant is K.(T.)=0.154 and the heat of reaction at a temperature T is given by AH _o()= a . (T.) +% 40,dr, (9) where AH.(T) = 57200 J/mol and AC,(T)=12.80 7.239x10-T +4.301x10-$T? +1.573x108T J/mol-K). Write a script file HW7_2_YourLastName.m that plots yno, as a function of temperature, yno, (T), over the temperature range of 200 to 400 K at P = 0.1, 1, and 10 bar. )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


