Question: please do this on matlab 2. The equation Cp = a + bT + cT-2 + dT3 is an empirical polynomial that describes the behavior
please do this on matlab
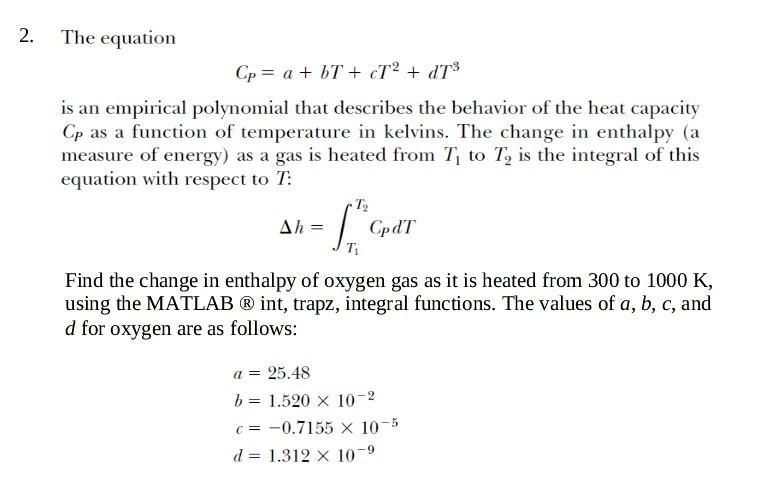
2. The equation Cp = a + bT + cT-2 + dT3 is an empirical polynomial that describes the behavior of the heat capacity Cp as a function of temperature in kelvins. The change in enthalpy (a measure of energy) as a gas is heated from Ti to T2 is the integral of this equation with respect to T: T2 Find the change in enthalpy of oxygen gas as it is heated from 300 to 1000 K, using the MATLAB int, trapz, integral functions. The values of a, b, c, and d for oxygen are as follows a 25.48 b= 1.520 10-2 c=-0.7155 10-5 d= 1.312 10-9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


