Question: please don't copy. I need an answer with a full explanation please help in understanding this problem. solve this problem only not earlier one In
please don't copy. I need an answer with a full explanation please help in understanding this problem.

solve this problem only not earlier one
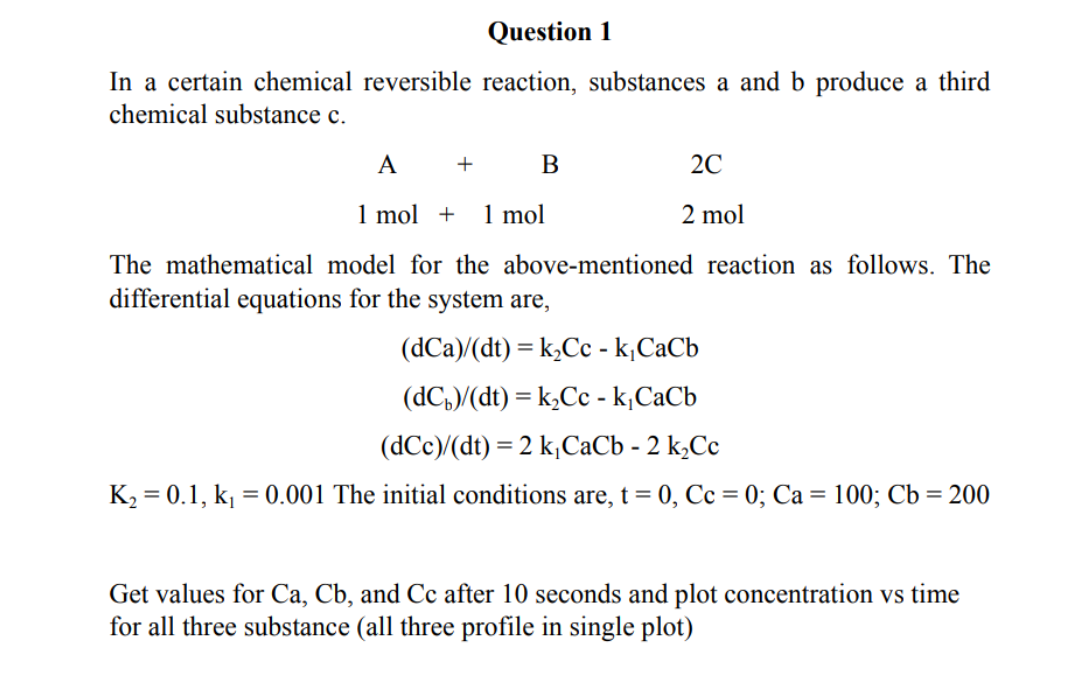
In a certain chemical reversible reaction, substances a and b produce a third chemical substance c. A+B2C1mol+1mol2mol The mathematical model for the above-mentioned reaction as follows. The differential equations for the system are, (dCa)/(dt)=k2Cck1CaCb(dCb)/(dt)=k2Cck1CaCb(dCc)/(dt)=2k1CaCb2k2Cc K2=0.1,k1=0.001 The initial conditions are, t=0,Cc=0;Ca=100;Cb=200 Get values for Ca,Cb, and Cc after 10 seconds and plot concentration vs time for all three substance (all three profile in single plot) FIND A RATE EQUATION USING THE INTEGRAL METHOD Reactant A decomposes in a batch reactor AProduct The composition of A in the reactor is measured at various times with results shown in the following columns 1 and 2. Find a rate equation (rA) to represent the data? A) Guess First-Order Kinetics, plot ln(CA0/CA) vs t and make comment B) Guess Second-Order Kinetics, plot (1/CA) vs t and make comment C) Guess n-Order Kinetics, find n and write rate equation that represent this data Note:- All calculations should be done by software (MATLAB, Excel, etc.) only
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


