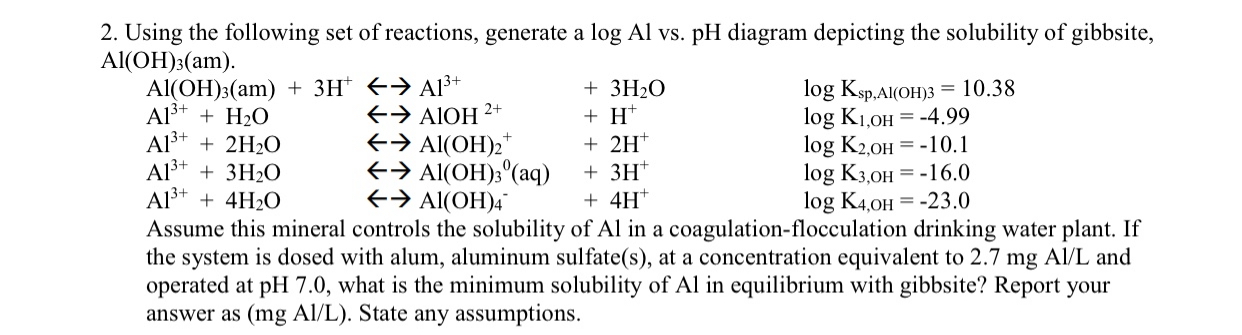
Question: ( PLEASE DONT FORGET THE GRAPH ) Using the following set of reactions, generate a l o g A l vs . p H diagram
PLEASE DONT FORGET THE GRAPH
Using the following set of reactions, generate a vs diagram depicting the solubility of gibbsite,
larr
larr
larr
larr
larr
Assume this mineral controls the solubility of in a coagulationflocculation drinking water plant. If the system is dosed with alum, aluminum sulfates at a concentration equivalent to mgA and operated at what is the minimum solubility of in equilibrium with gibbsite? Report your answer as mgA
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


