Question: Please DRAW out the mechanism and structures One challenge in the field of protein bioconjugation is the development of strategies to site-selectively modify a protein
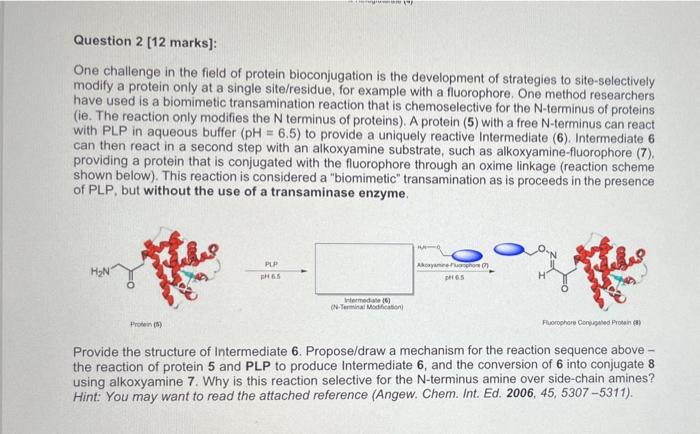
One challenge in the field of protein bioconjugation is the development of strategies to site-selectively modify a protein only at a single site/residue, for example with a fluorophore. One method researchers have used is a biomimetic transamination reaction that is chemoselective for the N-terminus of proteins (ie. The reaction only modifies the N terminus of proteins). A protein (5) with a free N-terminus can react with PLP in aqueous buffer (pH=6.5) to provide a uniquely reactive Intermediate (6). Intermediate 6 can then react in a second step with an alkoxyamine substrate, such as alkoxyamine-fluorophore (7). providing a protein that is conjugated with the fluorophore through an oxime linkage (reaction scheme shown below). This reaction is considered a "biomimetic" transamination as is proceeds in the presence of PLP, but without the use of a transaminase enzyme. Provide the structure of Intermediate 6. Propose/draw a mechanism for the reaction sequence above the reaction of protein 5 and PLP to produce Intermediate 6 , and the conversion of 6 into conjugate 8 using alkoxyamine 7. Why is this reaction selective for the N-terminus amine over side-chain amines? Hint: You may want to read the attached reference (Angew. Chem. Int. Ed. 2006, 45, 5307-5311)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


