Question: please dumb this down for me as much as possible The reform reaction between steam and gaseous methane (CH4) produces synthesis gas, a mixture of
 please dumb this down for me as much as possible
please dumb this down for me as much as possible
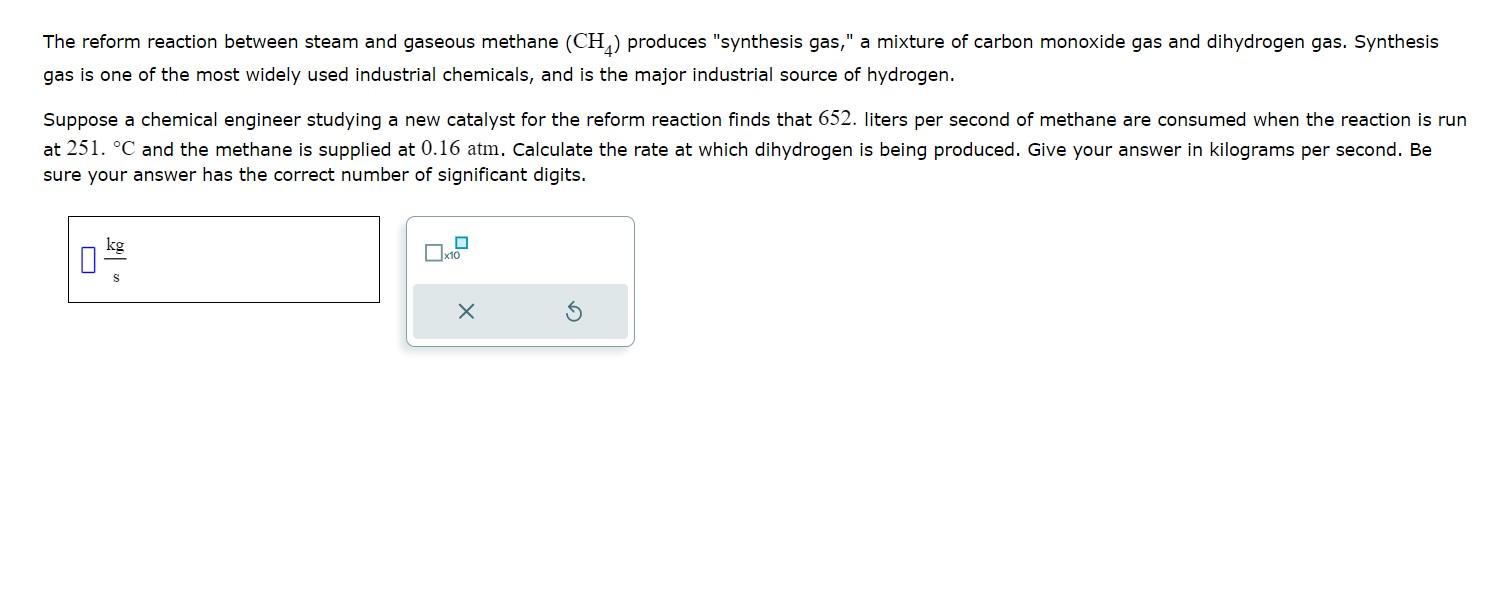
The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that 652 . liters per second of methane are consumed when the reaction is at 251.C and the methane is supplied at 0.16atm. Calculate the rate at which dihydrogen is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


