Question: please enclose the answer for each question thank you Consider a reaction with AH =20 kJ and AS = 60 J/K. Determine the AG for
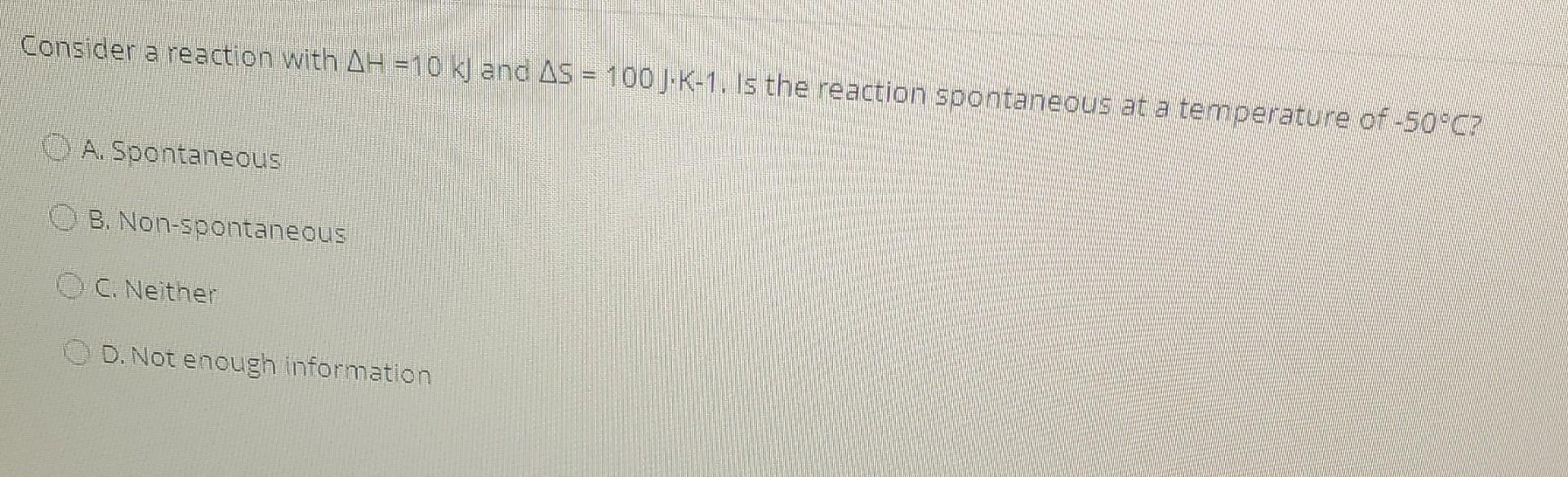
please enclose the answer for each question thank you
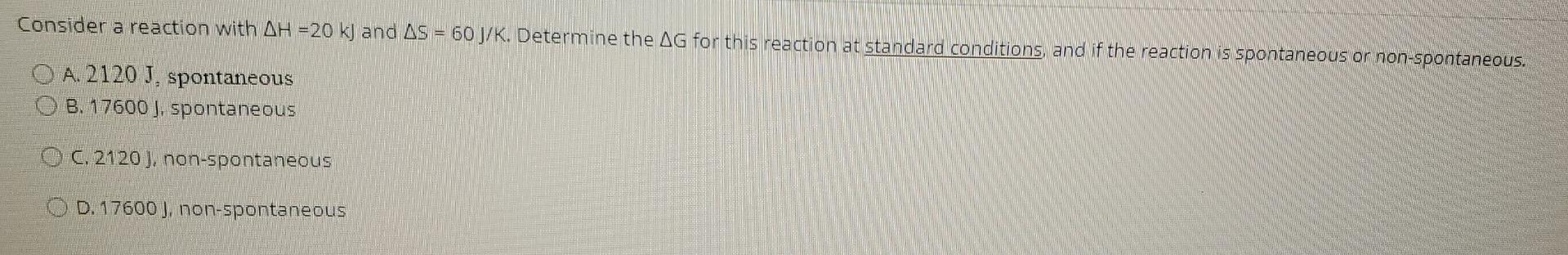
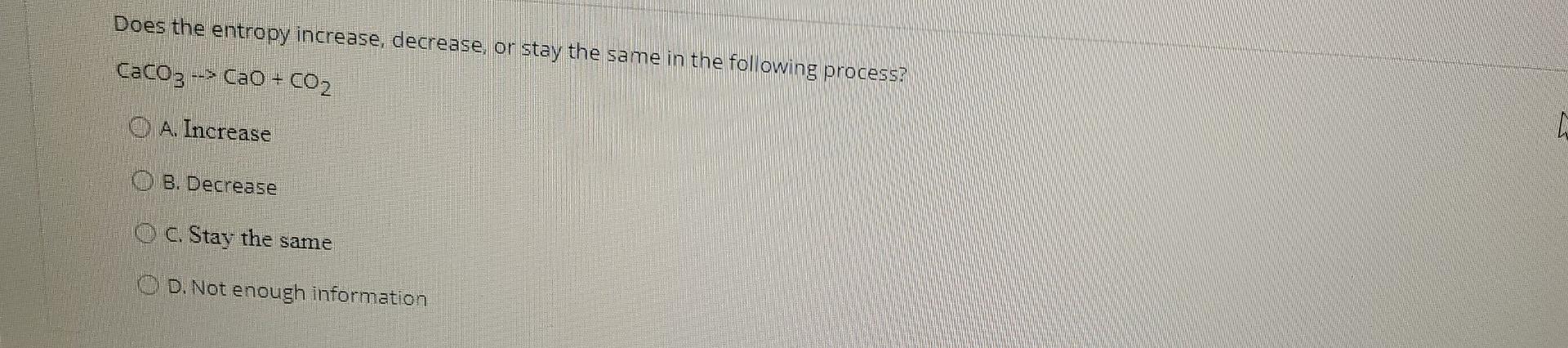
Consider a reaction with AH =20 kJ and AS = 60 J/K. Determine the AG for this reaction at standard conditions, and if the reaction is spontaneous or non-spontaneous. O A 2120 J. spontaneous O B. 17600 ), spontaneous O . 2120 J, non-spontaneous OD. 17600), non-spontaneous Does the entropy increase, decrease or stay the same in the following process? CaCO3 -- --> CaO + CO2 O A. Increase 8. Decrease C. Stay the same O D. Not enough information Consider a reaction with AH = 10 kj and AS = 100 J.K-1. Is the reaction spontaneous at a temperature of -50C? A. Spontaneous B. Non-spontaneous C. Neither D. Not enough information
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


