Question: Hello, Please answer question and make answer clear to understand. Constants | Periodic Table Part A Without doing any calculations, determine the sign of ASsysfor
Hello,
Please answer question and make answer clear to understand.
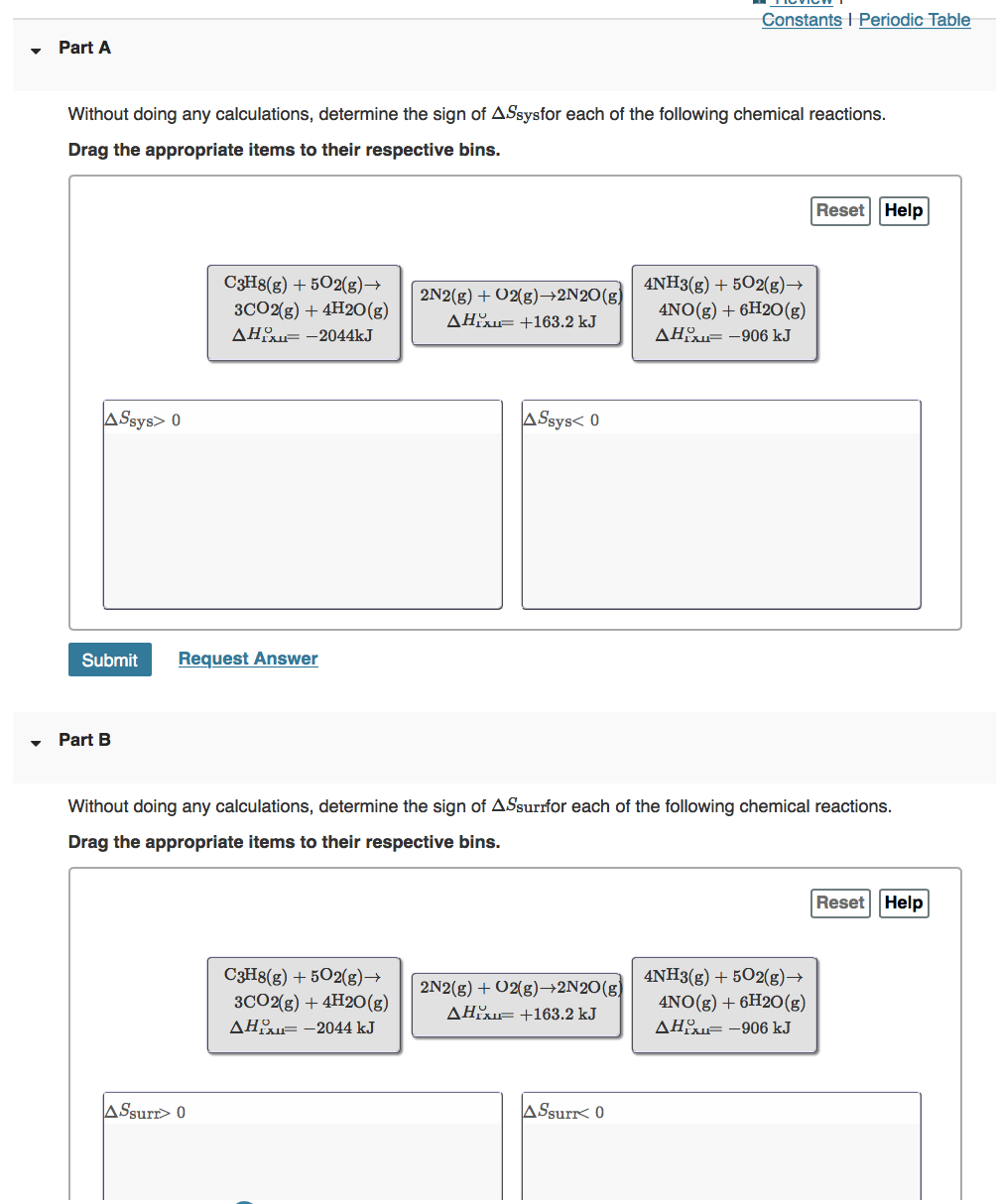
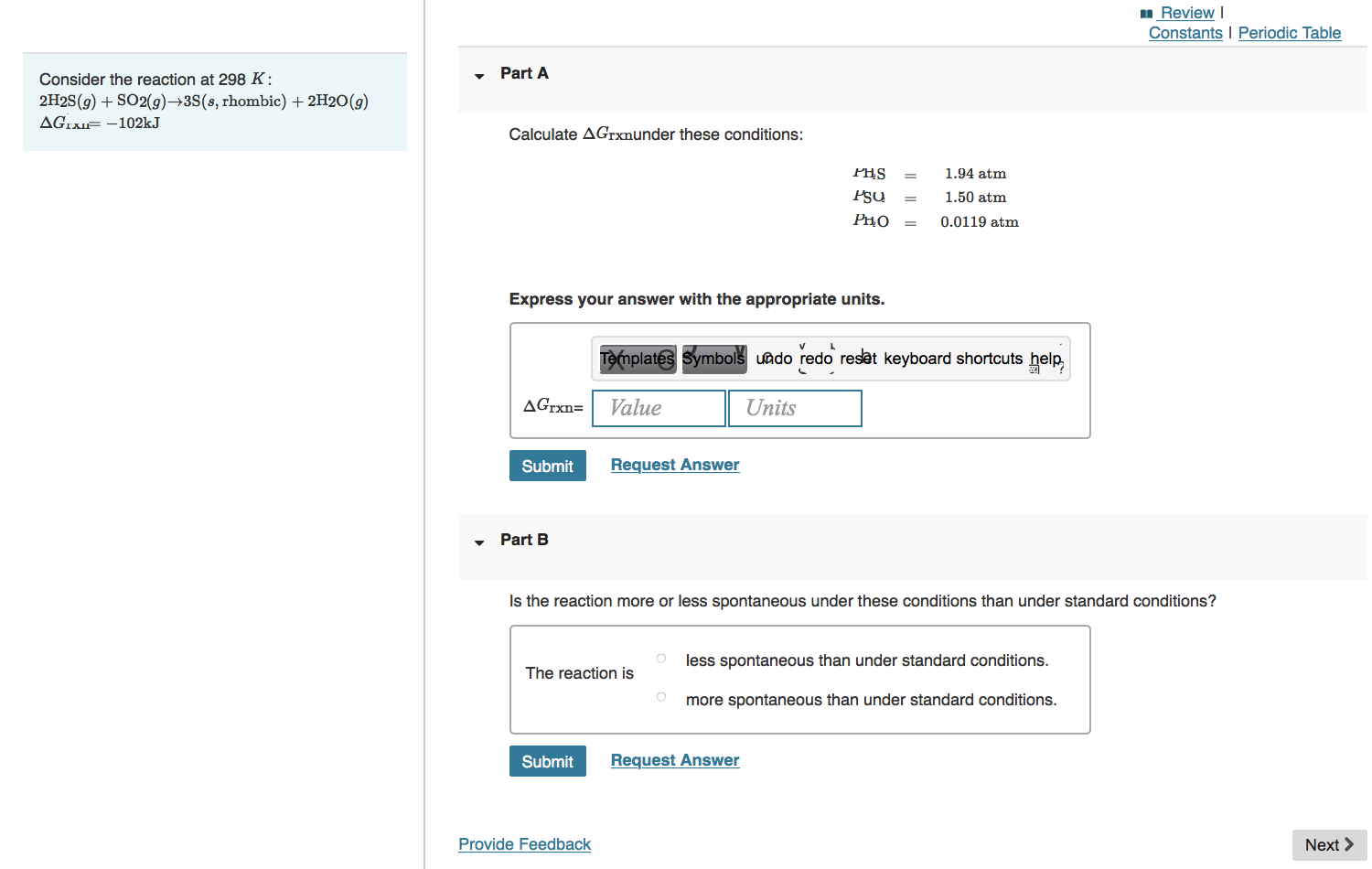
Constants | Periodic Table Part A Without doing any calculations, determine the sign of ASsysfor each of the following chemical reactions. Drag the appropriate items to their respective bins. Reset Help C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) AH-2044kJ 2N2(g) + O2(g)2N2O(g) AH +163.2 kJ 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) AH-906 kJ ASsys> 0 ASsys 0 Consider the reaction at 298 K: 2H2S(g) + SO2(g) 3S(s, rhombic) + 2H2O(g) AGTXI-102kJ Part A Calculate AGrxnunder these conditions: PHS Psu PHO = Express your answer with the appropriate units. AGrxn= Submit Review I Constants I Periodic Table 1.94 atm 1.50 atm 0.0119 atm Templates Symbols undo redo reset keyboard shortcuts help, Value Units Request Answer Part B Is the reaction more or less spontaneous under these conditions than under standard conditions? O less spontaneous than under standard conditions. The reaction is more spontaneous than under standard conditions. Submit Provide Feedback Request Answer Next >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


