Question: Please explain all steps At high temperatures, phosphine (PH3) dissociates into phosphorus and hydrogen by the following reaction: 4PH3P4+6H2 At 800C the rate at which
Please explain all steps
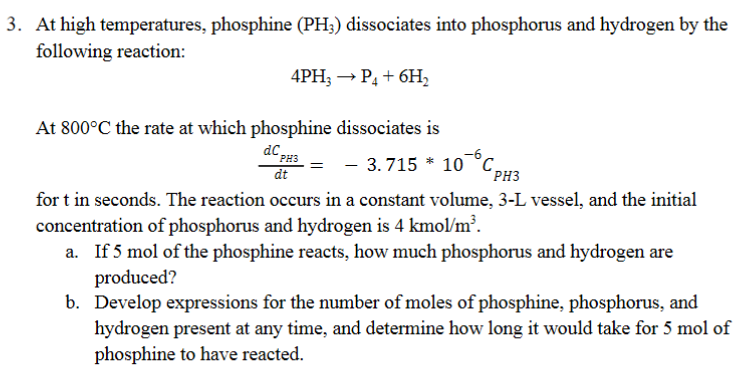
At high temperatures, phosphine (PH3) dissociates into phosphorus and hydrogen by the following reaction: 4PH3P4+6H2 At 800C the rate at which phosphine dissociates is dtdCPH3=3.715106CPH3 for t in seconds. The reaction occurs in a constant volume, 3 -L vessel, and the initial concentration of phosphorus and hydrogen is 4kmol/m3. a. If 5mol of the phosphine reacts, how much phosphorus and hydrogen are produced? b. Develop expressions for the number of moles of phosphine, phosphorus, and hydrogen present at any time, and determine how long it would take for 5mol of phosphine to have reacted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


