Question: please explain and answer a,b,c,d,e, & f. thanks A student mixes 5mL0.002MFe(NO3)3 in 1MHNO3 with 1mL0.002MKSCN and 4mL of water. She finds that in the
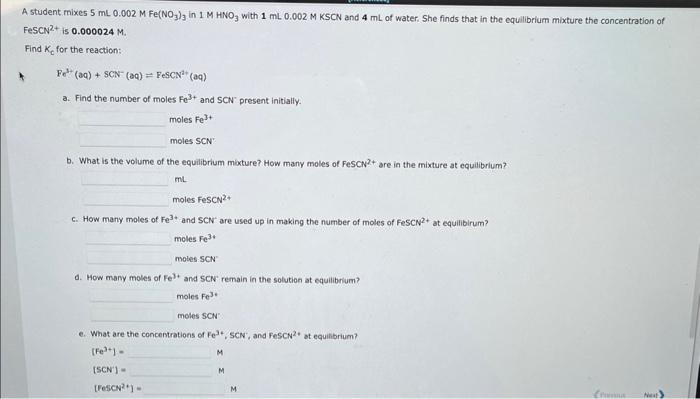

A student mixes 5mL0.002MFe(NO3)3 in 1MHNO3 with 1mL0.002MKSCN and 4mL of water. She finds that in the equilibrium mixture the concentration of FesCN2+ is 0.000024M Find Kc for the reaction: Fe3(aq)+SCN(aq)=FeSCN2+(aa) a. Find the number of moles Fe3+ and SCN present initially. molesFe3+molesSCN: b. What is the volume of the equilibrium moxture? How many moles of FescN? + are in the mixture at equilibrium? molesFeSCN2+ c. How many moles of Fe3+ and SCN2 are used up in making the number of moles of FeSCN+ at equilibirum? molesFe3+moles:SCN d. How many moles of Fe 3+ and SCN remain in the solution at equilibrium? molesFe3-moles5CN 6. What are the concentrations of Fe3+,SCN; and FeSCN2 t at equilibrium? [Fe3+]=[SCN]=[[escses+]=M b. What is the volume of the equilibrium mixture? How many moles of FeSCN 2+ are in the mixture at equilibrium? mLmolesFeSCN2+ c. How many moles of Fe3+ and SCN are used up in making the number of moles of FeSCN 2+ at equilibirum? molesFe3+molesSCN d. How many moles of Fe3+ and SCNremain in the solution at equilibrium? molesFe3+ moles5CN. e. What are the concentrations of Fe3+,SCN2, and FeSCN2+ at equilibrium? [Fe3+]=[SCN]]=M[FeSCN2+]=M f. What is the value of Kc for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


