Question: Please explain and correct this problem. Thank you! Page 3 (6 pts)3. A student performs an experiment by adding 10.0 mL of concentrated HCI (12.0
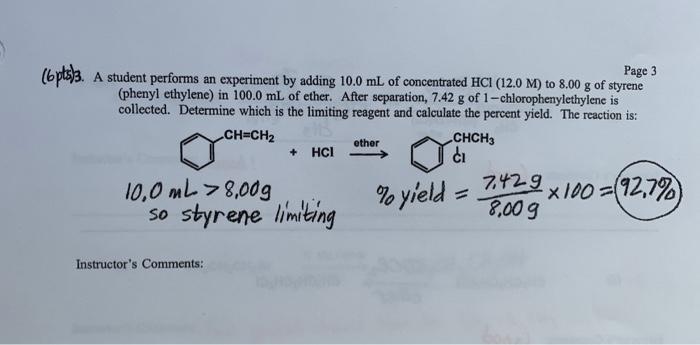
Page 3 (6 pts)3. A student performs an experiment by adding 10.0 mL of concentrated HCI (12.0 M) to 8.00 g of styrene (phenyl ethylene) in 100.0 mL of ether. After separation, 7.42 g of 1-chlorophenylethylene is collected. Determine which is the limiting reagent and calculate the percent yield. The reaction is: CH=CH2 CHCH3 + HCl ci 7429 10,0 mL > 8,00g % yield x100=(92,7% so styrene limiting 8,009 ether Instructor's Comments
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


