Question: Please explain and show solution. Thank you - Your Turn 1. At 327C, the equil concentrations for the reaction: CH3OH(g)CO(g)+2H2(g) are [CH3OH]=0.15M,[CO]=0.24M and [H2]=1.1 M.
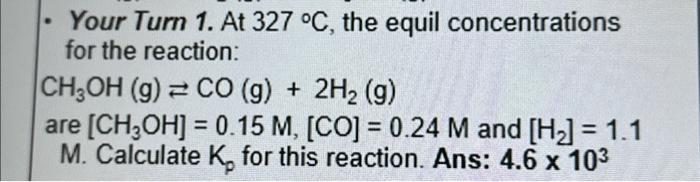
- Your Turn 1. At 327C, the equil concentrations for the reaction: CH3OH(g)CO(g)+2H2(g) are [CH3OH]=0.15M,[CO]=0.24M and [H2]=1.1 M. Calculate Kp for this reaction. Ans: 4.6103
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


