Question: Please explain Constants It takes 53 0 J to raise the temperature of an 8 40 g piece of unknown metal from 13.0 C to
Please explain
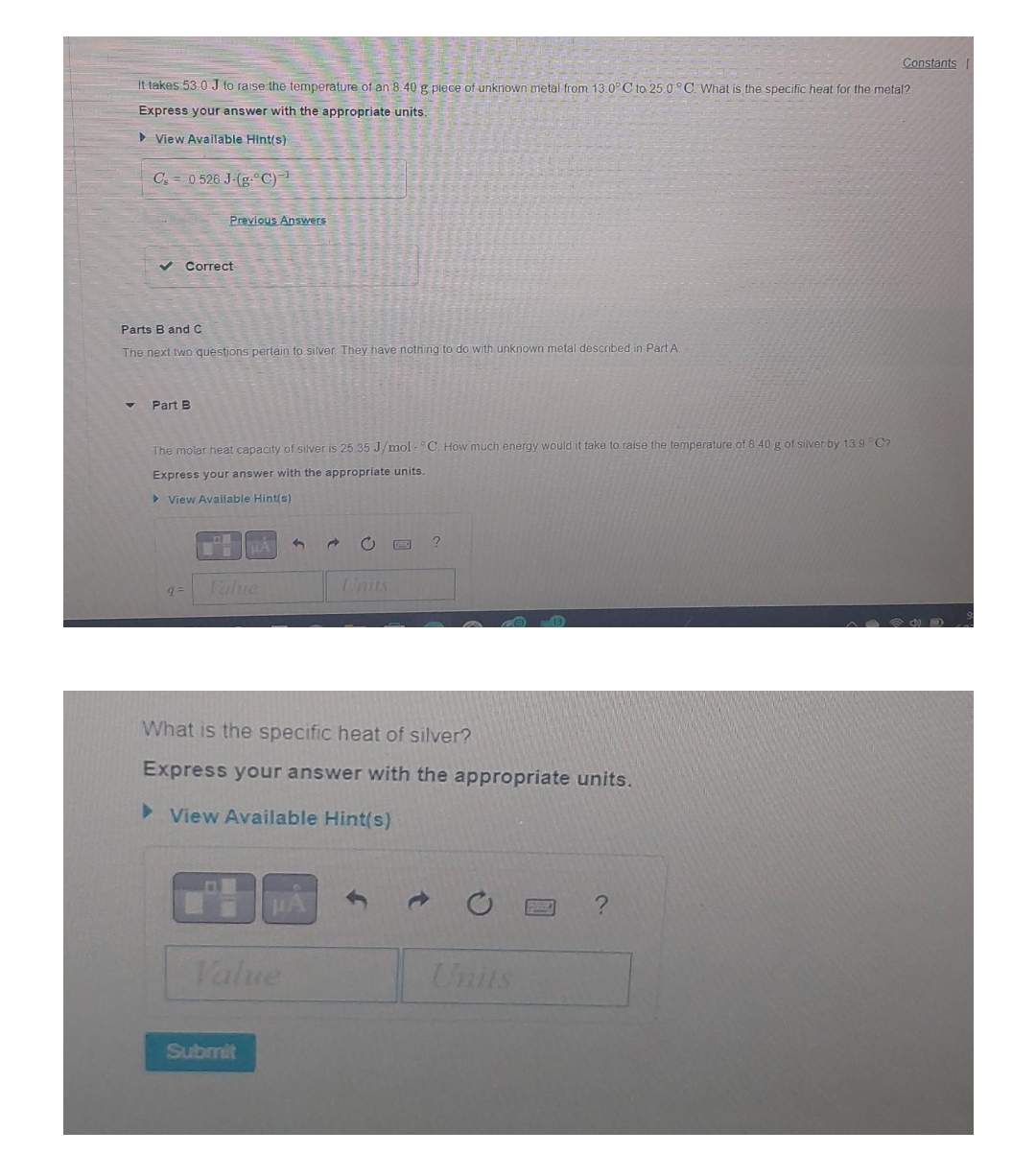
Constants It takes 53 0 J to raise the temperature of an 8 40 g piece of unknown metal from 13.0 C to 25.0 C. What is the specific heat for the metal? Express your answer with the appropriate units. View Available Hint(s) C's = 0 526 J.(g."C) Previous Answers Correct Parts B and C The next two questions pertain to silver. They have nothing to do with unknown metal described in Part A. Part B The molar heat capacity of silver is 25.35 J/mol . "C. How much energy would it take to raise the temperature of 8 40 g of silver by 13.9 . Co Express your answer with the appropriate units View Available Hint(s) ? 9= Value Units What is the specific heat of silver? Express your answer with the appropriate units. View Available Hint(s) O D ? Value Units Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


