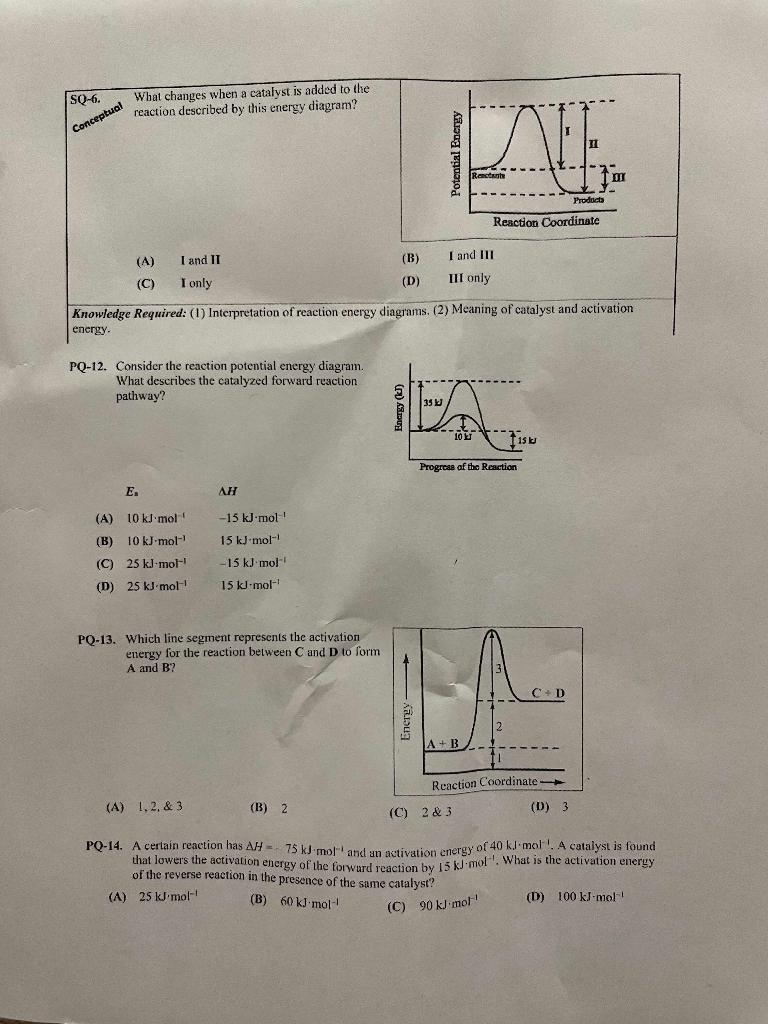
Question: Please explain each problem. Thanks SQ-6. What changes when a catalyst is added to the reaction described by this energy diagram? (A) I and II
 Please explain each problem. Thanks
Please explain each problem. Thanks
SQ-6. What changes when a catalyst is added to the reaction described by this energy diagram? (A) I and II (B) I and III (C) I only (D) III only Knowledge Required: (1) Interpretation of reaction energy diagrams. (2) Meaning of catalyst and activation energy. PQ-12. Consider the reaction potential energy diagram. What describes the catalyzed forward reaction pathway? (A) 10kJmol115kJmol1 (C) 25kJmol15kJ1mol1 (D) 25kJmol115kJmol1 PQ-13. Which line segment represents the activation energy for the reaction between C and D to form A and B ? (A) 1,2,&3 (B) 2 (C) 2&3 (D) 3 PQ-14. A certain reaction has H=75kJmol1 and an activation energy of 40k.1mol1.4 catalyst is found that lowers the activation energy of the forward reaction by 15kJmol1. What is the activation energy of the reverse reaction in the presence of the sume catalyst? (A) 25kJmmol1 (B) 60kJmmol1 (D) 100kJmol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


