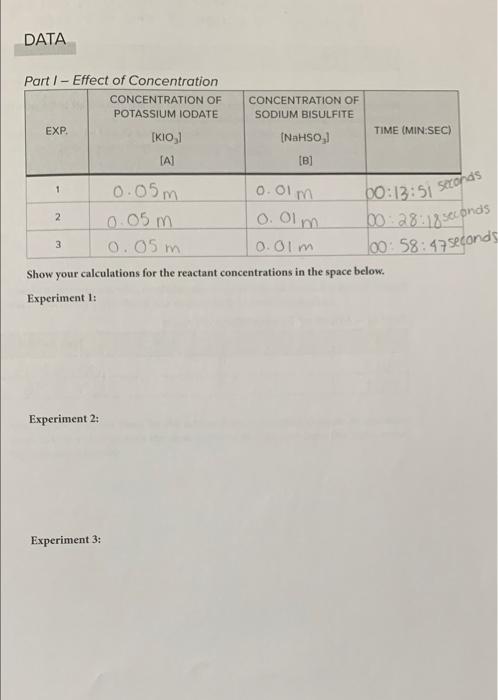
Question: please help!! DATA Part I - Effect of Concentration CONCENTRATION OF CONCENTRATION OF POTASSIUM IODATE SODIUM BISULFITE EXP TIME (MIN:SEC) [KIO,1 (NaHSO, IA] [B] 0.05
![OF POTASSIUM IODATE SODIUM BISULFITE EXP TIME (MIN:SEC) [KIO,1 (NaHSO, IA] [B]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d13dcd4cb_52566f8d13d75133.jpg)

DATA Part I - Effect of Concentration CONCENTRATION OF CONCENTRATION OF POTASSIUM IODATE SODIUM BISULFITE EXP TIME (MIN:SEC) [KIO,1 (NaHSO, IA] [B] 0.05 m 0.0lm 2 0.05m o. Olm 3 0.05m 0.0lm Show your calculations for the reactant concentrations in the space below. Experiment : 1 00:13:51 seconds 00:28:18 seconds 100:58:47 seconds Experiment 2: Experiment 3: Part II - Effect of a Catalyst What effect should the addition of a catalyst have on the rate of the reaction? - A catalyst includes anolher pathway of Tower activation energy: Hence a catalyst increases the rate of the reaction IA) 18] TIME (MIN SEC) 00:8:13 seconds How do the results compare between this run and the first run in Part I? Do your results support your answer given at the beginning of Part II? TIME (MIN SEC) Part III - Effect of Temperature on Rate TEMP. [A] [B] cold not 00:40:55 00: 7.96 QUESTIONS 1. Describe the results of your experiment in Part I. Did changing the concentration of either reactant have any effect on the rate? Explain. 2. Using your data for how the reaction time changes, calculate a rate law for the reaction using the equation given. Show your work below. um) Times 3. Based on your rate law, what would happen to the rate if you doubled only [A]? What if you doubled only [B]? What if you doubled both [A] and [B]? Explain. 4. How did the reaction times for the cold and warm reactions compare to the initial room temperature trial with the same amounts of reactants? Does this make sense? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


