Question: please explain each step thank you Reactive metals such as magnesium react readily with acids in aqueous solution. 2.17g of Mg is added to 81.4mL
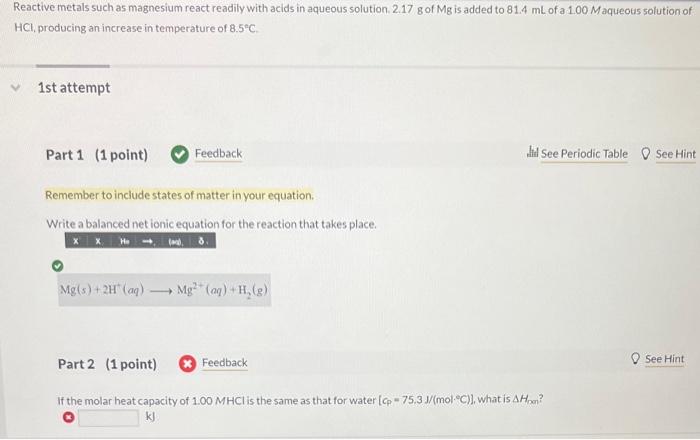
Reactive metals such as magnesium react readily with acids in aqueous solution. 2.17g of Mg is added to 81.4mL of a 1.00Maqueous solution of HCl, producing an increase in temperature of 8.5C. 1st attempt Part 1 (1 point) Feedback Wit See Periodic Table 0 See Hint Remember to include states of matter in your equation. Write a balanced net ionic equation for the reaction that takes place. Mg(s)+2H+(aq)Mg2+(aq)+H2(g) Part 2 (1 point) Feedback If the molar heat capacity of 1.00MHCl is the same as that for water [cp=75.3J/(molC)], what is Hpen ? kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


