Question: Please explain, give correct answer D Question 5 5 pts A metal pellet with a mass of 15.5 grams is heated then dropped into 31.0
Please explain, give correct answer
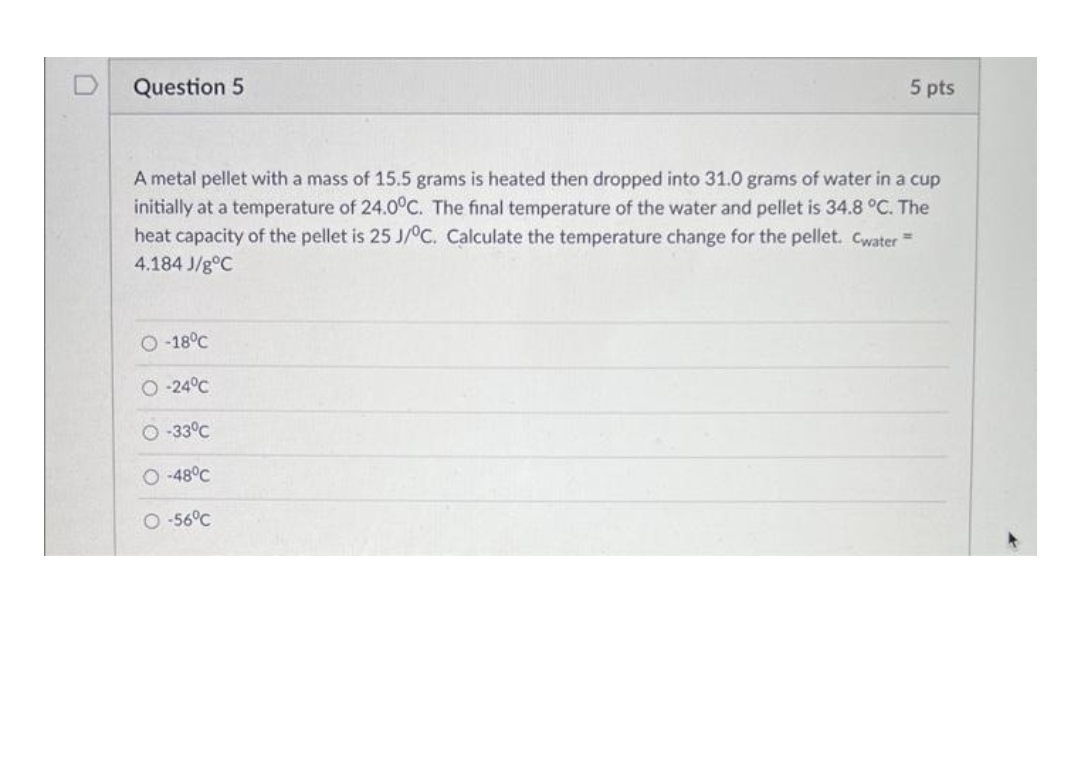
D Question 5 5 pts A metal pellet with a mass of 15.5 grams is heated then dropped into 31.0 grams of water in a cup initially at a temperature of 24.00C. The final temperature of the water and pellet is 34.8 .C. The heat capacity of the pellet is 25 J/C. Calculate the temperature change for the pellet. Cwater = 4.184 J/g C -18c -240C -330C O -48C O -56C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


