Question: Please explain how to work out the balances. I am struggling to workout the a and e. The growth of baker's yeast (Saccharomyces cerevisiae) on
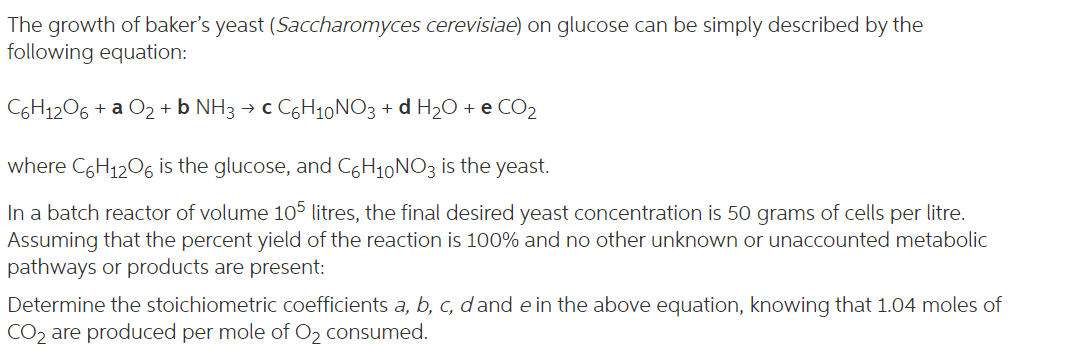 Please explain how to work out the balances. I am struggling to workout the a and e.
Please explain how to work out the balances. I am struggling to workout the a and e.
The growth of baker's yeast (Saccharomyces cerevisiae) on glucose can be simply described by the following equation: C6H12O6 + a O2 + NH3 + c C6H10 NO3 + d H20 + e CO2 + where C6H12O6 is the glucose, and C6H10NO3 is the yeast. In a batch reactor of volume 105 litres, the final desired yeast concentration is 50 grams of cells per litre. Assuming that the percent yield of the reaction is 100% and no other unknown or unaccounted metabolic pathways or products are present: Determine the stoichiometric coefficients a, b, c, d and e in the above equation, knowing that 1.04 moles of CO2 are produced per mole of Oz consumed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


