Question: please explain how you reached the answer if possible :) The simplified mechanism shown below is for the thermal decomposition of ethanal: CH3CHOk1CH3+.CHOCH3+CH3CHOk2CH4+CH3COCH3COk3CO+CH3CH3+CH3k4C2H6 (i) Draw
please explain how you reached the answer if possible :)
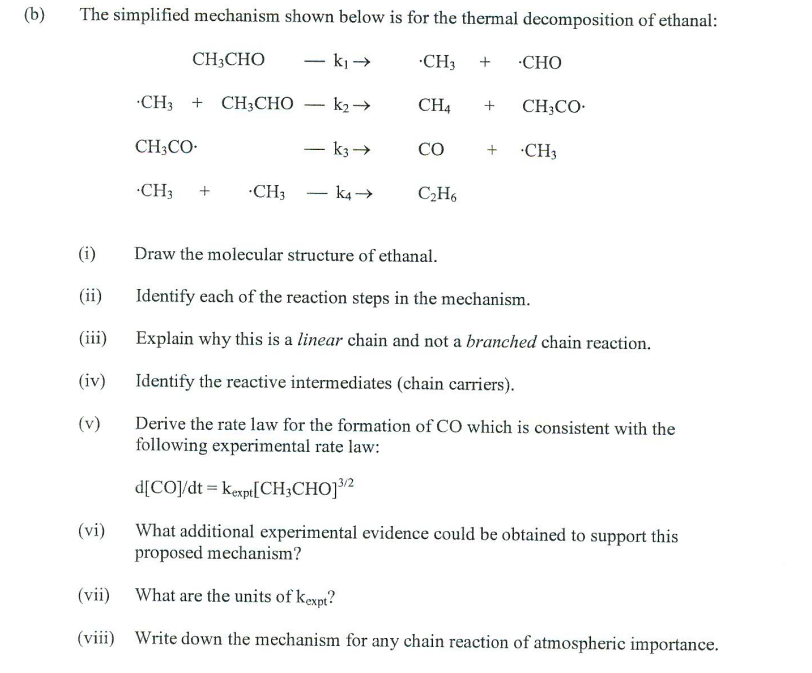
The simplified mechanism shown below is for the thermal decomposition of ethanal: CH3CHOk1CH3+.CHOCH3+CH3CHOk2CH4+CH3COCH3COk3CO+CH3CH3+CH3k4C2H6 (i) Draw the molecular structure of ethanal. (ii) Identify each of the reaction steps in the mechanism. (iii) Explain why this is a linear chain and not a branched chain reaction. (iv) Identify the reactive intermediates (chain carriers). (v) Derive the rate law for the formation of CO which is consistent with the following experimental rate law: d[CO]/dt=kexpt[CH3CHO]3/2 (vi) What additional experimental evidence could be obtained to support this proposed mechanism? (vii) What are the units of kexpt ? (viii) Write down the mechanism for any chain reaction of atmospheric importance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


