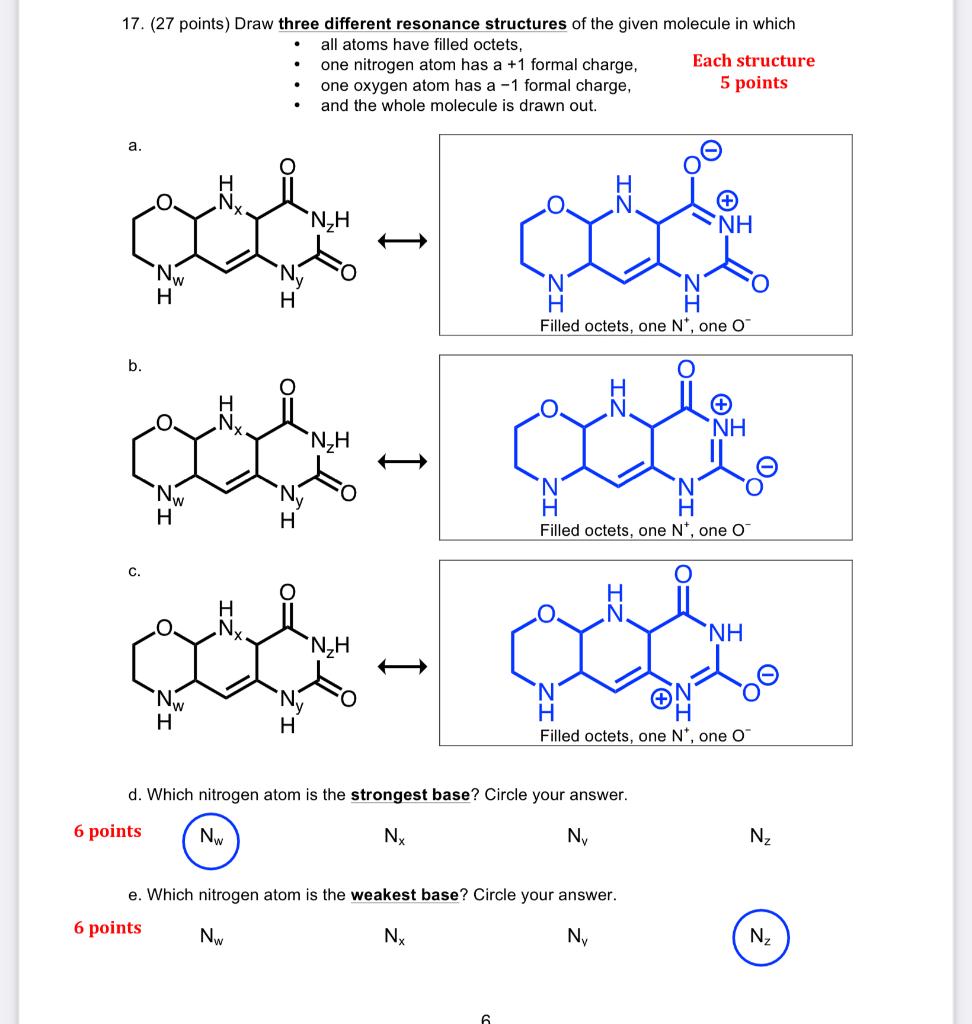
Question: please explain question d and e 17. (27 points) Draw three different resonance structures of the given molecule in which - all atoms have filled
 please explain question d and e
please explain question d and e
17. (27 points) Draw three different resonance structures of the given molecule in which - all atoms have filled octets, - one nitrogen atom has a + 1 formal charge, Each structure - one oxygen atom has a 1 formal charge, 5 points - and the whole molecule is drawn out. a. Filled octets, one N+, one O b. d. Which nitrogen atom is the strongest base? Circle your answer. 6 points Nx NV Nz e. Which nitrogen atom is the weakest base? Circle your answer. 6 points NW NX NV Nz
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


