Question: Give the electronic configuration of the oxygen (O) atom. Show it both as an electron-populated orbital diagram and as a list of orbitals with
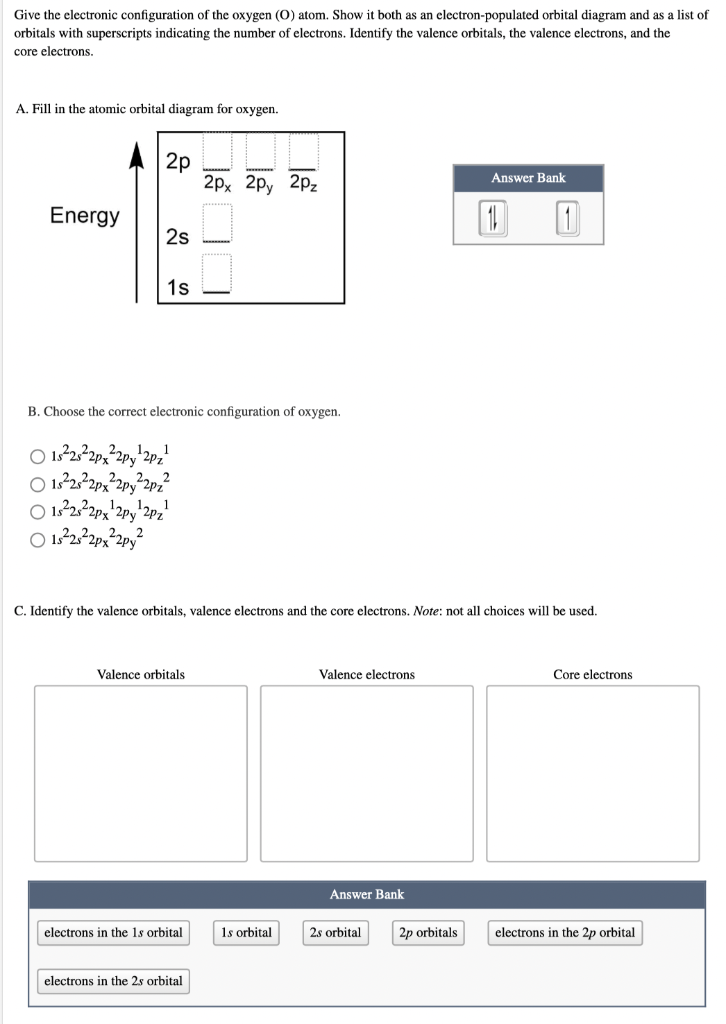
Give the electronic configuration of the oxygen (O) atom. Show it both as an electron-populated orbital diagram and as a list of orbitals with superscripts indicating the number of electrons. Identify the valence orbitals, the valence electrons, and the core electrons. A. Fill in the atomic orbital diagram for oxygen. Energy 2p 2s 1s B. Choose the correct electronic configuration of oxygen. 182s2px2py2pz 1s2s2px2py2pz 152s2px 2py 2pz 1s2s2px2py Valence orbitals 1 1 electrons in the 1s orbital 2px 2px 2pz electrons in the 2s orbital C. Identify the valence orbitals, valence electrons and the core electrons. Note: not all choices will be used. 1s orbital Valence electrons Answer Bank 2s orbital Answer Bank 2p orbitals 1 1 Core electrons electrons in the 2p orbital
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
A B 16 Q afom 8 15 25 ap 15 25 85 16 0 atom 15 as apx ... View full answer

Get step-by-step solutions from verified subject matter experts


