Question: please explain steps clearly. thank you 29) Calculate the atomic mass of chromium if chromium has four naturally occurring isotopes with the 29) following masses
please explain steps clearly.
thank you 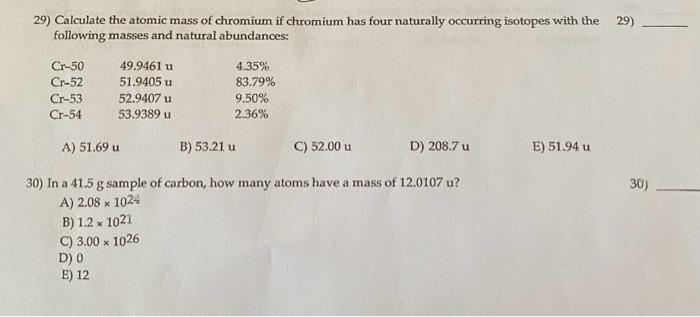

29) Calculate the atomic mass of chromium if chromium has four naturally occurring isotopes with the 29) following masses and natural abundances: Cr50Cr52Cr53Cr5449.9461u51.9405u52.9407u53.9389u4.35%83.79%9.50%2.36% A) 51.69u B) 53.21u C) 52.00u D) 208.7u E) 51.94u 30) In a 41.5g sample of carbon, how many atoms have a mass of 12.0107u? 30i) A) 2.081024 B) 1.21021 C) 3.001026 D) 0 E) 12
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


