Question: Please explain thank you! PFe(0) PFe+0, 3. Consider the interchange of O2 and CO coordinated to myoglobin, an iron porphyrin denoted as PFe: ; PFe
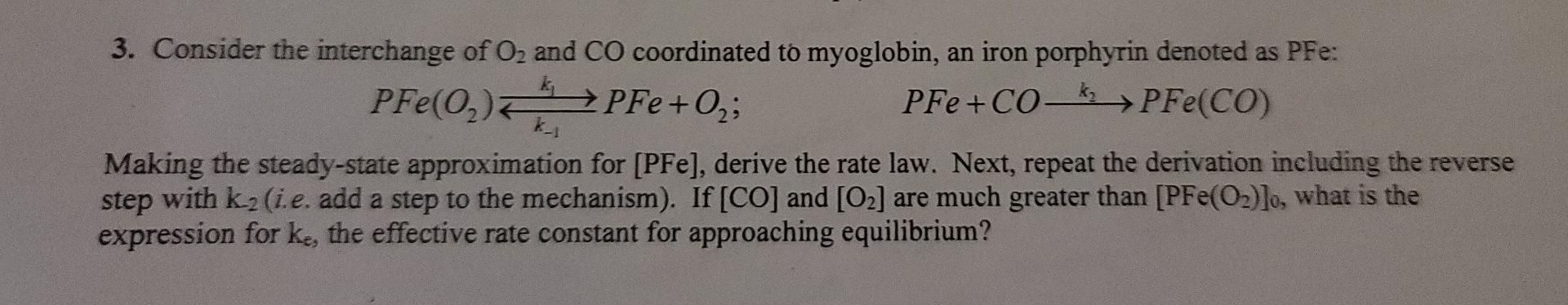
Please explain thank you!
PFe(0) PFe+0, 3. Consider the interchange of O2 and CO coordinated to myoglobin, an iron porphyrin denoted as PFe: ; PFe +CO PFe(CO) k_1 Making the steady-state approximation for [PFe), derive the rate law. Next, repeat the derivation including the reverse step with k.(i.e. add a step to the mechanism). If [CO] and [O2] are much greater than [PFe(O2)]o, what is the expression for ke, the effective rate constant for approaching equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


