Question: please explain the hard soft acid base theory behind the reaction. is ethyl iodide hard or soft? is ethyl tosylate hard or soft? how does
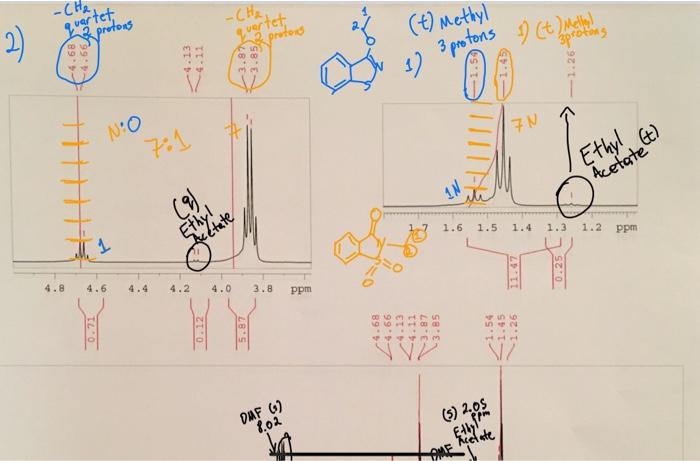
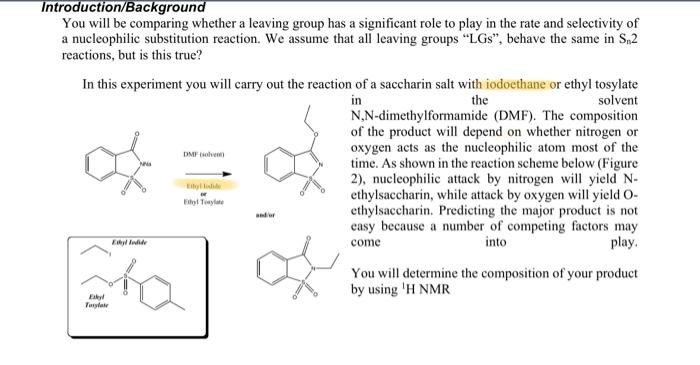
-Che, quartet protons 2 2 -CHA, vertet protond -4.666 (t) Methyl 12 1 (t) Melly! sprotons EI 3 protons 0000 + MM 7N NO 7:1 Acetate (t) (8). rectate 1.6 1.5 1.4 1.3 1.2 Ethyl ppm 11.47 4.8 4.6 4.4 4.2 4.0 3.8 ppm un MO 0.12 5.87 VY NA (5) 2.08 DAF G 1.02 Date ME Introduction/Background You will be comparing whether a leaving group has a significant role to play in the rate and selectivity of a nucleophilic substitution reaction. We assume that all leaving groups "LGs, behave the same in S.2 reactions, but is this true? In this experiment you will carry out the reaction of a saccharin salt with iodoethane or ethyl tosylate in the solvent N,N-dimethylformamide (DMF). The composition of the product will depend on whether nitrogen or oxygen acts as the nucleophilic atom most of the time. As shown in the reaction scheme below (Figure 2), nucleophilic attack by nitrogen will yield N- ethylsaccharin, while attack by oxygen will yield O- El Taylor ethylsaccharin. Predicting the major product is not easy because a number of competing factors may come into play. You will determine the composition of your product by using 'H NMR DMF Esby Todd ha F ust
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


