Question: PLEASE EXPLAIN This chapter continues to study allylic carbocations, specifically, benzylic carbocations. Because the positive charge can be delocalized to multiple sites, they are highly
PLEASE EXPLAIN
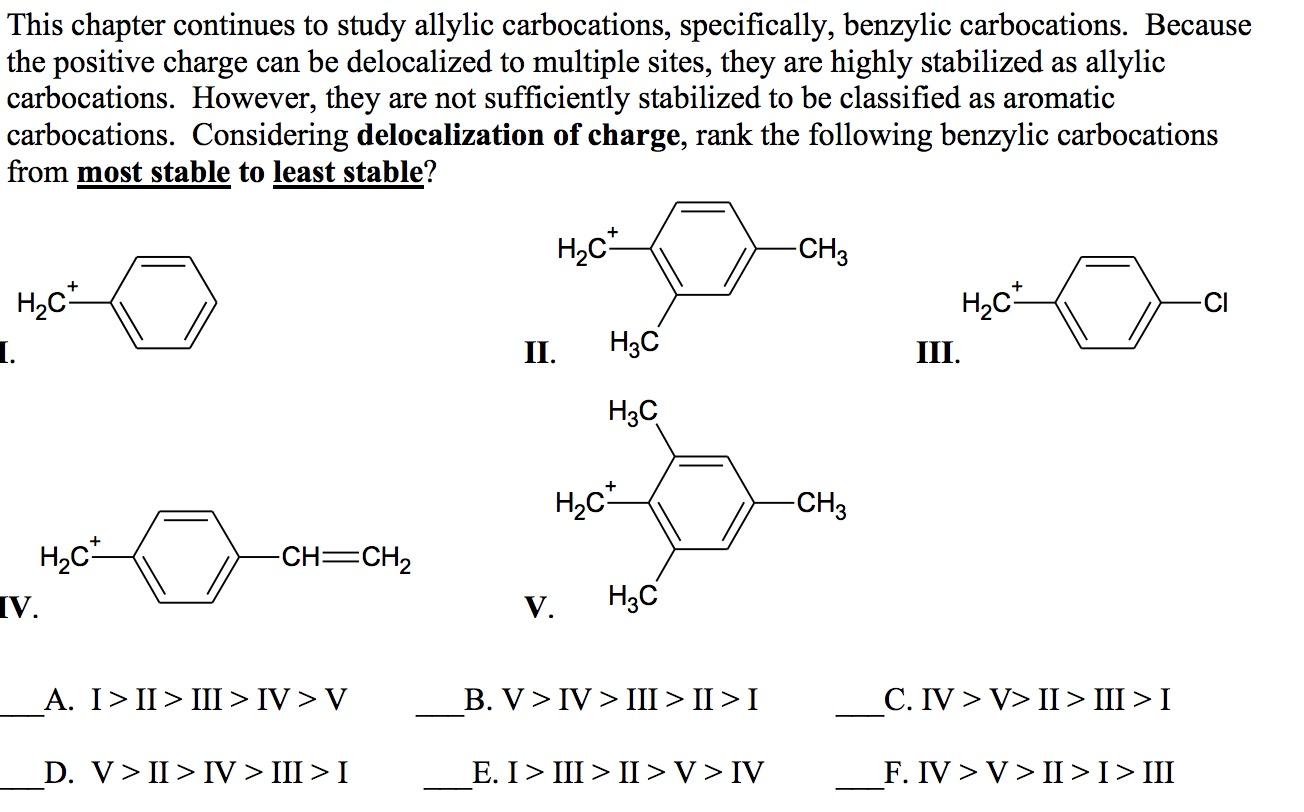
This chapter continues to study allylic carbocations, specifically, benzylic carbocations. Because the positive charge can be delocalized to multiple sites, they are highly stabilized as allylic carbocations. However, they are not sufficiently stabilized to be classified as aromatic carbocations. Considering delocalization of charge, rank the following benzylic carbocations from most stable to least stable? H.C -CH3 H2C+ H2C 1. o CI II. H3C III. H3C H.C* -CH3 H.ch -CH=CH2 V. V. . A. I>II > III > IV>V B. V > IV > III > II >I C. IV>V> II > III >I D. V > II > IV > III > I E. I > III > II >V> IV F. IV>V > II > I >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


